Probenecid
Probenecid, also sold under the brand name Probalan, is a medication that increases uric acid excretion in the urine. It is primarily used in treating gout and hyperuricemia.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Probalan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682395 |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75-95% |
| Elimination half-life | 2-6 hours (dose: 0.5-1 g) |
| Excretion | renal (77-88%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.313 |
| Chemical and physical data | |
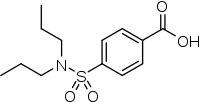
| Formula | C13H19NO4S |
| Molar mass | 285.36 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Probenecid was developed as an alternative to caronamide[1] to competitively inhibit renal excretion of some drugs, thereby increasing their plasma concentration and prolonging their effects.
Medical uses
Probenecid is primarily used to treat gout and hyperuricemia.
Probenecid is sometimes used to increase the concentration of some antibiotics and to protect the kidneys when given with cidofovir. Specifically, a small amount of evidence supports the use of intravenous cefazolin once rather than three times a day when it is combined with probenecid.[2]
It has also found use as a masking agent,[3] potentially helping athletes using performance-enhancing substances to avoid detection by drug tests.
Adverse effects
Mild symptoms such as nausea, loss of appetite, dizziness, vomiting, headache, sore gums, or frequent urination are common with this medication. Life-threatening side effects such as thrombocytopenia, leukemia and encephalopathy are extremely rare.[4] Theoretically probenecid can increase the risk of uric acid kidney stones.
Drug interactions
Some of the important clinical interactions of probenecid include those with captopril, indomethacin, ketoprofen, ketorolac, naproxen, cephalosporins, quinolones, penicillins, methotrexate, zidovudine, ganciclovir, lorazepam, and acyclovir. In all these interactions, the excretion of these drugs is reduced due to probenecid.[5] This could lead to excessive levels of these drugs building up, causing toxicity.
Pharmacology
Probenecid probably has several pharmacological targets, including blocking pannexins.[6] Probenecid is also useful in the treatment of gout where the mechanism of action is believed to be focused on the kidney. Probenecid interferes with the kidneys' organic anion transporter (OAT), which reclaims uric acid from the urine and returns it to the plasma.[7] If probenecid (an organic acid) is present, the OAT binds preferentially to it (instead of to uric acid), preventing reabsorption of the uric acid. Hence, the urine retains more uric acid, lowering uric acid concentration in the plasma. This is a good example of a medical usage for competition between substrates transported across cell membranes. This same effect, however, alters excretion of acidic drugs by the kidney, leading to the many drug interactions noted above.
Pharmacokinetics
In the kidneys, probenecid is filtered at the glomerulus, secreted in the proximal tubule and reabsorbed in the distal tubule.
History
During World War II, probenecid was used to extend limited supplies of penicillin; this use exploited probenecid's interference with drug elimination (via urinary excretion) in the kidneys and allowed lower doses of penicillin to be used.[8]
See also
References
- MASON RM (June 1954). "Studies on the Effect of Probenecid ('Benemid') in Gout". Ann. Rheum. Dis. 13 (2): 120–30. doi:10.1136/ard.13.2.120. PMC 1030399. PMID 13171805.
- Cox, VC; Zed, PJ (March 2004). "Once-daily cefazolin and probenecid for skin and soft tissue infections". The Annals of Pharmacotherapy. 38 (3): 458–63. doi:10.1345/aph.1d251. PMID 14970368.
- Morra V, Davit P, Capra P, Vincenti M, Di Stilo A, Botrè F (December 2006). "Fast gas chromatographic/mass spectrometric determination of diuretics and masking agents in human urine: Development and validation of a productive screening protocol for antidoping analysis". J Chromatogr A. 1135 (2): 219–29. doi:10.1016/j.chroma.2006.09.034. PMID 17027009.
- Kydd AS, Seth R, Buchbinder R, Edwards CJ, Bombardier C (November 14, 2014). "Uricosuric medications for chronic gout". Cochrane Database Syst Rev (11): CD010457. doi:10.1002/14651858.CD010457.pub2. PMID 25392987.
- Cunningham RF, Israili ZH, Dayton PG (March–April 1981). "Clinical pharmacokinetics of probenecid". Clin Pharmacokinet. 6 (2): 135–51. doi:10.2165/00003088-198106020-00004. PMID 7011657.CS1 maint: date format (link)
- Silverman W, Locovei S, Dahl G (September 2008). "Probenecid, a gout remedy, inhibits pannexin 1 channels". Am. J. Physiol., Cell Physiol. 295 (3): C761–7. doi:10.1152/ajpcell.00227.2008. PMC 2544448. PMID 18596212.
- Hsyu PH, Gisclon LG, Hui AC, Giacomini KM (January 1988). "Interactions of organic anions with the organic cation transporter in renal BBMV". Am. J. Physiol. 254 (1 Pt 2): F56–61. doi:10.1152/ajprenal.1988.254.1.F56. PMID 2962517.
- Butler D (2005). "Wartime tactic doubles power of scarce bird-flu drug". Nature. 438 (7064): 6. doi:10.1038/438006a. PMID 16267514.