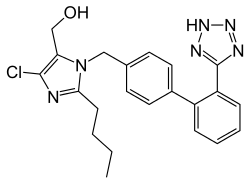
Losartan
Losartan, sold under the trade name Cozaar among others, is a medication mainly used to treat high blood pressure.[2] It is also used for diabetic kidney disease, heart failure, and left ventricular enlargement.[2] It is taken by mouth.[2] It may be used alone or in addition to other blood pressure medication.[2] Up to six weeks may be required for the full effects to occur.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /loʊˈsɑːrtən/ |
| Trade names | Cozaar, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695008 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Angiotensin II receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25–35% |
| Protein binding | 99.7% (primarily albumin) |
| Metabolism | Liver (CYP2C9, CYP3A4) |
| Elimination half-life | 1.5–2 hours |
| Excretion | Kidney 13–25%, biliary 50–60% |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.555 |
| Chemical and physical data | |
| Formula | C22H23ClN6O |
| Molar mass | 422.91 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common adverse effects include muscle cramps, stuffy nose, cough, high blood potassium and anemia.[2] Severe adverse effects may include angioedema, low blood pressure, and kidney problems.[2] Use during pregnancy may result in harm to the baby.[2][1] Use is not recommended during breastfeeding.[1] It is in the angiotensin receptor blocker family of medication. It works by blocking angiotensin II.[2]
Losartan was patented in 1986, and approved for medical use in the United States in 1995.[2][3] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[4] It is available as a generic medication.[5] The wholesale cost in the developing world is about US$0.28–3.45 per month as of 2015.[6] In the United States, as of 2017, the wholesale cost of a typical dose is $1.13 per month.[7] In 2016, it was the 9th most prescribed medication in the United States, with more than 49 million prescriptions.[8] A version combined with hydrochlorothiazide is available.[2]
Medical uses
Losartan is used for hypertension, including in people with left ventricular hypertrophy (enlarged heart muscle), and kidney dysfunction among type II diabetics.[9] It may also delay progression of diabetic nephropathy. It is a suitable pharmacological agent for the reduction of renal disease progression in patients with type 2 diabetes, hypertension, and microalbuminuria (>30 mg/24 hours) or proteinuria (>900 mg/24 hours).[10]
Although evidence shows calcium channel blockers and thiazide-type diuretics are preferred first-line treatments for most people (due to both efficacy and cost), an angiotensin II receptor antagonist such as losartan is recommended as first-line treatment in people under the age of 55 who cannot tolerate an ACE inhibitor.[11] One study demonstrated losartan was superior to atenolol in the primary prevention of adverse cardiovascular events (myocardial infarction or stroke), with a reduction in cardiovascular morbidity and mortality for a comparable reduction in blood pressure. The maximal effects on blood pressure usually occur within 3–6 weeks of starting losartan.[12]
Adverse effects
The most common adverse effects for losartan in adults are upper respiratory infections, dizziness, and back pain.[13] People with type 2 diabetes and kidney disease may experience diarrhea, fatigue, low blood pressure, low blood glucose, elevated potassium, chest pain, or allergic reaction.[13] Losartan should not be taken by people who are diabetic and taking aliskiren.[14] Anemia may occur, due to inhibition of the renin–angiotensin system.[15] As with other angiotensin receptor blockers, losartan may injure the liver, although this effect appears to be rare.[16] Electrolyte imbalances may occur in people taking losartan with kidney problems.[9] Adverse outcomes do not differ by gender, age or race.[9]
Pregnancy
In October 2014, the U.S. Food and Drug Administration (FDA) issued a black box warning that losartan can cause fetal toxicity, and should be discontinued as soon as pregnancy is detected.[17][14] Using losartan while pregnant could result in fetal injury or death.[17][14]
Overdose
Overdosing would most likely result in decreased blood pressure, which could manifest as an increased heart rate, dizziness, feeling light headed, or loss of consciousness. Mice studies showed that lethality occurred at about 44 to 170 times the maximum recommended dose after the mice weight were adjusted.[9]
Interactions
Losartan may have adverse interactions with phenobarbital, rifampin, or fluconazole, possibly inhibiting its blood pressure-lowering effects.[9]
Contamination
Between November 2018, and September 2019, the FDA announced multiple recalls of tablets containing losartan by Sandoz, Torrent Pharmaceuticals, Hetero Labs, Camber Pharmaceuticals, Legacy Pharmaceutical Packaging, Teva Pharmaceuticals, Vivimed Life Sciences, and Macleods Pharmaceutical Limited due to detection of one of the possible carcinogens N-nitrosodiethylamine, N-methylnitrosobutyric acid, or N-nitroso-N-methyl-4-aminobutyric acid in the active pharmaceutical ingredient (API).[18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]
Mechanism of action
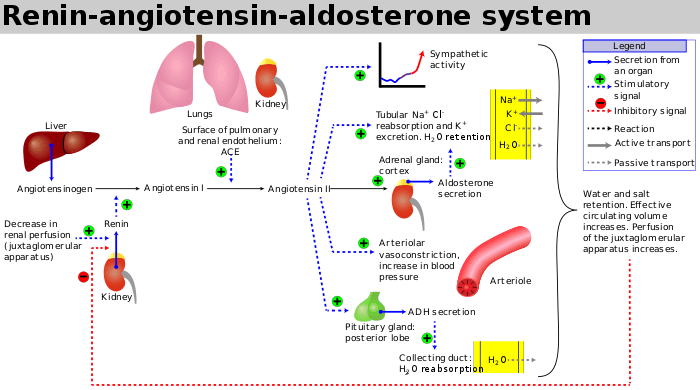
Losartan is a selective, competitive angiotensin II receptor type 1 (AT1) antagonist, reducing the end organ responses to angiotensin II. Losartan administration results in a decrease in total peripheral resistance (afterload) and cardiac venous return (preload). All of the physiological effects of angiotensin II, including release of aldosterone, are antagonized in the presence of losartan. Reduction in blood pressure occurs independently of the status of the renin–angiotensin system. As a result of losartan dosing, plasma renin activity increases due to removal of the angiotensin II feedback. Renin is released from the kidneys when there is reduced renal arterial pressure, sympathetic activation, or increased sodium delivery to the distal renal tubule.[36] Renin then acts by converting angiotensinogen to angiotensin I; angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II; angiotensin II causes vasoconstriction and aldosterone release.[36] Aldosterone serves to retain sodium from the distal renal tubule. Sodium retention ultimately results in increased blood pressure.[37] Therefore, the use of angiotensin II receptor antagonists like losartan result in blocking the downstream effect of renin, angiotensin II, and ultimately decreasing blood pressure.
Angiotensin II receptor antagonists include losartan, valsartan, azilsartan, candesartan, eprosartan, irbesartan, olmesartan, and telmisartan. They all have the same mechanism of action and potentially inhibit the actions of angiotensin better than ACE inhibitors, such as lisinopril, because there are other enzymes than ACE that have the capability of producing angiotensin II.[36]
Losartan is a uricosuric. As a specific inhibitor of the urate transporter 1 (SLC22A12, URAT1), losartan blocks the uptake of uric acid into cells, thus leaving more available in the bloodstream to be filtered and excreted by the kidneys.[38] Because losartan can cause hyperkalemia, individuals should not use potassium supplements or salt substitutes containing potassium without appropriate monitoring by a physician.[39]
Pharmacokinetics
Losartan is well absorbed following oral administration and undergoes significant first-pass metabolism to produce the 5-carboxylic acid metabolite, designated as EXP3174. About 14% of an oral dosage is converted to this metabolite, which is long-acting (6 to 8 hr) and a noncompetitive antagonist at the AT1 receptor, contributing to the pharmacological effects of losartan. EXP3174 is 10-40 times more potent in blocking AT1 receptors than losartan. In addition, the binding to the target enzyme is pH-sensitive, and the negatively-charged tetrazole ring, which is similar in size to the negative carboxylic acid derivative, may contribute to the activity of the drug.[40]
Losartan's bioavailability is about 32%.
Metabolism is primarily by cytochrome P450 isoenzymes CYP2C9 and CYP3A4. Peak plasma concentrations of losartan and EXP3174 occur about one hour and three to four hours, respectively, after an oral dose. Both losartan and EXP3174 are more than 98% bound to plasma proteins. Losartan is excreted in the urine, and in the feces via bile, as unchanged drug and metabolites. About 4% of an oral dose is excreted unchanged in urine, and about 6% is excreted in urine as the active metabolite. The terminal elimination half lives of losartan and EXP3174 are about 1.5 to 2.5 hours and 3 to 9 hours, respectively.
Losartan and other angiotensin-receptor antagonists exhibit fetal toxicity and should be avoided during pregnancy, particularly in the second and third trimesters.[41]
Chemistry
Losartan is generally marketed as the (basic) potassium salt of the aromatized negatively charged tetrazole, called "losartan potassium".[42]
Dosing
The suggested initial dose of losartan for treatment of hypertension is 50 mg/day, and the usual maintenance dose range is 25–100 mg/day.[36] There is limited data on what dosage is considered toxic in humans; toxicity would most likely result in decreased blood pressure and an increased heart rate.[9]
References
- "Losartan (Cozaar) Use During Pregnancy". Drugs.com. Retrieved 10 December 2017.
- "Losartan Potassium". The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 470. ISBN 9783527607495.
- "World Health Organization model list of essential medicines: 21st list 2019". 2019. hdl:10665/325771. Cite journal requires
|journal=(help) - British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 127. ISBN 9780857111562.
- "Single Drug Information". International Medical Products Price Guide. Retrieved 9 December 2017.
- "NADAC as of 2017-12-06". Centers for Medicare and Medicaid Services. Retrieved 10 December 2017.
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- "cozaar - FDA" (PDF). FDA.gov. September 2008. Retrieved 11/5/2019. Check date values in:
|access-date=(help) - Boersma C, Atthobari J, Gansevoort RT, de Jong-Van den Berg LT, de Jong PE, de Zeeuw D, Annemans LJ, Postma MJ (2006). "Pharmacoeconomics of angiotensin II antagonists in type 2 diabetic patients with nephropathy: implications for decision making". PharmacoEconomics. 24 (6): 523–35. doi:10.2165/00019053-200624060-00001. PMID 16761901.
- "Hypertension in adults: diagnosis and management". National Institute for Health and Care Excellence (NICE). Retrieved 8 April 2017.
- Abrams A (2007). 'Clinical Drug Therapy Rationales for Nursing Practice. Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 846. ISBN 978-0-7817-6263-2.
- "Patient Information Cozaar" (PDF). Merck. December 2015. Retrieved 8 April 2017.
- Merck & Co., Inc. (January 2014). "Cozaar" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 8 April 2017.
- Cheungpasitporn, W; Thongprayoon, C; Chiasakul, T; Korpaisarn, S; Erickson, SB (November 2015). "Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis". QJM : Monthly Journal of the Association of Physicians. 108 (11): 879–84. doi:10.1093/qjmed/hcv049. PMID 25697787.

- Patti R, Sinha A, Sharma S, Yoon TS, Kupfer Y (May 2019). "Losartan-induced severe hepatic injury: A case report and literature review". Cureus. 11 (5): e4769. doi:10.7759/cureus.4769. PMC 6663042. PMID 31363450.
- "Cozaar (losartan potassium) 25 mg, 50 mg, and 100 mg Tablets". U.S. Food and Drug Administration (FDA). 16 October 2014. Archived from the original on 12 January 2017. Retrieved 21 July 2015.
- "FDA provides update on its ongoing investigation into ARB drug products; reports on finding of a new nitrosamine impurity in certain lots of losartan and product recall" (Press release). U.S. Food and Drug Administration (FDA). 3 October 2019. Archived from the original on 3 October 2019. Retrieved 3 October 2019.
- "Sandoz Inc. Issues Voluntary Nationwide Recall of One Lot of Losartan Potassium and Hydrochlorothiazide Due to the Detection of Trace Amounts of NDEA (N-Nitrosodiethylamine) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 8 November 2018. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP". U.S. Food and Drug Administration (FDA). 20 December 2018. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP". U.S. Food and Drug Administration (FDA). 3 January 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "UPDATED: Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium and Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 22 January 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Updated: Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium /Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 1 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Updated: Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium/Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 18 April 2019. Archived from the original on 6 October 2019. Retrieved 5 October 2019.
- "Updated: Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium / Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 23 September 2019. Archived from the original on 6 October 2019. Retrieved 23 September 2019.
- "Legacy Pharmaceutical Packaging, LLC Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 50mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 15 July 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- "Macleods Pharmaceutical Limited Issues Voluntary Nationwide Consumer Level Recall of Losartan Potassium 50mg and Losartan Potassium/Hydrochlorothiazide combination Tablets 50mg/12.5mg, 100mg/12.5mg and 100mg/25mg due to detection of NMBA (N-Nitroso-N-Methyl-4-aminobutyric acid) Impurity". U.S. Food and Drug Administration (FDA). 26 June 2019. Archived from the original on 1 September 2019. Retrieved 5 October 2019.
- "Teva Pharmaceuticals USA, Inc. Expands Voluntary Nationwide Recall of Losartan Potassium to 50 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply, Inc". U.S. Food and Drug Administration (FDA). 11 June 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- "Vivimed Life Sciences Pvt Ltd Issues Voluntary Nationwide Recall of Losartan Potassium 25 mg, 50 mg and 100 mg Tablets, USP Due to the Detection of Trace Amounts of N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) Impurity". U.S. Food and Drug Administration (FDA). 3 May 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- "Teva Pharmaceuticals USA, Inc. Issues Voluntary Nationwide Recall of Losartan Potassium 25 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply". U.S. Food and Drug Administration (FDA). 26 April 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25mg, 50mg, And 100mg Due to The Detection of Trace Amounts Of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) Impurity Found in The Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 19 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25mg, 50mg, and 100mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 28 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 50mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 15 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Camber Pharmaceuticals, Inc. Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25 mg, 50 mg and 100 mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 28 February 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- "Macleods Pharmaceuticals Limited Issues Voluntary Nationwide Consumer Level Recall of One Lot (BLM 715A) of Losartan Potassium/Hydrochlorothiazide Combination Tablets 100mg/25mg Due to detection of NDEA (N-Nitrosodiethylamine) Impurity". U.S. Food and Drug Administration (FDA). 22 February 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- Katzung, Bertram G., editor. Basic & clinical pharmacology. ISBN 9781259641152. OCLC 1048625746.CS1 maint: multiple names: authors list (link) CS1 maint: extra text: authors list (link)
- Graudal, Niels A.; Hubeck-Graudal, Thorbjørn; Jürgens, Gesche (January 2012). "Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review)". American Journal of Hypertension. 25 (1): 1–15. doi:10.1038/ajh.2011.210. ISSN 1941-7225. PMID 22068710.
- Hamada T, Ichida K, Hosoyamada M, Mizuta E, Yanagihara K, et al. (1 October 2008). "Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients". American Journal of Hypertension. 21 (10): 1157–1162. doi:10.1038/ajh.2008.245. PMID 18670416.
- RxList. The Internet Drug Index. Clinical pharmacology of Cozaar. Retrieved January 6, 2014.
- Binding of losartan
- Sica DA, Gehr TW, Ghosh S (2005). "Clinical pharmacokinetics of losartan". Clin Pharmacokinet. 44 (8): 797–814. doi:10.2165/00003088-200544080-00003. PMID 16029066.
- see negatively charged tetrazole structure