Zucapsaicin
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. It is applied three times daily for a maximum of three months. Zucapsaicin is a member of phenols and a member of methoxybenzenes[1] It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions.[2][3] It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use (patch and cream).[4][5]
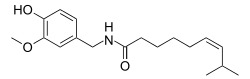 | |
| Clinical data | |
|---|---|
| Trade names | Civanex |
| Other names | Civamide; (Z)-Capsaicin; cis-Capsaicin |
| Routes of administration | Topical |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.164.527 |
| Chemical and physical data | |
| Formula | C18H27NO3 |
| Molar mass | 305.41188 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Zucapsaicin has been tested for treatment of a variety of conditions associated with ongoing nerve pain. This includes herpes simplex infections; cluster headaches and migraine; and knee osteoarthritis.[6] It was approved by the Health Canada in 2010 as topical cream marketed under the brand name Zuacta but currently not FDA-approved.[2] It has a melting point of 71.5–74.5.[2]
Pharmacology
Zucapsaicin mediates an antinociceptive action via acting as an agonist at TRPV1. TRPV1 play an important physiological role of transducing chemical, mechanical and thermal stimuli as well as pain transduction, and participate in pain modulation and perception. They are mainly distributed in C sensory nerve fibers as well as Aẟ fibers to transmit sensory information involving inflammatory and neuropathic pain, and activation of these channels releasesomatostatin, calcitonin gene-related peptide (CGRP) and other neuropeptides (neurokinin A, kassinin), leading to neurogenic inflammation [A19720]. Zucapsaicin is also reported to affect the peptidergic afferent neurons via a desensitization mechanism to decrease the levels of dorsal root ganglia and sciatic calcitonin gene-related peptide (CGRP) and substance P (SP) [L877].[2]
Pharmacodynamics
Zucapsaicin mediates an antinociceptive action via acting as an agonist at TRPV1. TRPV1 play an important physiological role of transducing chemical, mechanical and thermal stimuli as well as pain transduction, and participate in pain modulation and perception. They are mainly distributed in C sensory nerve fibers as well as Aẟ fibers to transmit sensory information involving inflammatory and neuropathic pain, and activation of these channels releasesomatostatin, calcitonin gene-related peptide (CGRP) and other neuropeptides (neurokinin A, kassinin), leading to neurogenic inflammation.[5] Zucapsaicin is also reported to affect the peptidergic afferent neurons via a desensitization mechanism to decrease the levels of dorsal root ganglia and sciatic calcitonin gene-related peptide (CGRP) and substance P (SP).[2]
Mechanism of action
Zucapsaicin excites and desensitizes C-fibers via agonist at TRPV1 on nociceptive neurons. It binds to intracellular sites and initially stimulates the channels, causing burning sensation.[3] The mechanism of pharmacological action of zucapsaicin has not been fully understood yet. It is suggested that this compound, similarly to its trans isomer, is an agonist of the vanilloid receptor VR1 (TRPV1) and a neuronal calcium channel blocker.[7][8] Capsaicin is able to excite and desensitize C-fibers. As such, it is not only able to cause pain, but also exhibit analgesic properties. Initially, it stimulates TRPV1, which is responsible for a burning sensation. This effect is followed by a longlasting refractory state – ‘desensitization’ – during which the previously excited sensory neurons become unresponsive to capsaicin and other stimuli. It was shown that desensitization and tachyphylaxis of TRPV1 channels contribute to capsaicin-induced pain relief.[9] Desensitization of TRPV1 represents the main mechanism of its inhibitory function.
Three distinct pathways of capsaicin-induced desensitization have been described: i) activation of calcineurin, which results in dephosphorylation of TRPV1; ii) activation of phospholipase C with the subsequent phosphatidylinositol 4,5-biphosphate hydrolysis (rather controversial) and iii) activation of calcium-dependent protein kinase C isoforms and subsequent channel phosphorylation.[10][11] Desensitization involves both tachyphylaxis (short-term desensitization) and long-term, persistent, desensitization.[12][13][14] It is suggested that the downregulation of proalgesic substances (such as SP) and upregulation of analgesic peptides are implicated in desensitization.[15] The exhaustion of SP reserves renders neurons desensitized and refractory. These mechanisms of desensitization are not fully understood. It is thought that the short-term desensitization is related to capsaicin’s ability to block the intra-axonal transport of NGF, SP and somatostatin.[16]
The desensitization is a reversible phenomenon. It begins a few hours after capsaicin application and may last even several weeks.[15] The reversible desensitization was found useful in the treatment of pain, whereas the site-specific ablation of sensory nerves transmitting pain stimuli is a promising approach (‘molecular scalpel’) to achieve a permanent pain relief in patients suffering from bone cancer pain or HIV-induced neuropathies.[12][13] Desensitization and depletion of pronociceptive neurotransmitters induce chemical denervation with a loss of function, which is clinically used in osteoarthritis, diabetic neuropathy, psoriasis and others.[17][18][19][20] In dorsal root ganglia and the sciatic nerve, zucapsaicin decreases levels of SP and CGRP, indicating that it influences peptidergic afferent neurons via a desensitization mechanism[21][41]. When administered topically, the intended targets for zucapsaicin are the neurons that innervate the local area of application. These neurons transmit pain toward the CNS.
Pharmacokinetics
Absorption
Zucapsaicin displays low systemic absorption and localizes at the area of application. In animal studies, systemic absorption is 0.075%.[22][23][24]
Metabolism
In vitro studies demonstrates weak to moderate inhibitiory effects on various cytochrome P450 enzymes, although not clinically significant due to low systemic absorption.[23]
Toxicity
Most common adverse effects involved application site reactions such as transient burning and warm sensation. Other adverse effects observed in clinical trials are eye irritation, arthralgia, aggravated osteoarthritis, burning sensation, headache, cough and sneezing. Oral LD50 in mouse is >87.5 mg/kg in male and <60 mg/kg in females. Oral LD50 in rats is >90 mg/kg in males and >60 mg/kg in females.[22]
Chemical and Physical Properties
Computed Properties[25]
| Property Name | Property Value |
|---|---|
| Molecular Weight | 305.418 g/mol |
| XLogP3-AA | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 9 |
| Exact Mass | 305.199 g/mol |
| Monoisotopic Mass | 305.199 g/mol |
| Topological Polar Surface Area | 58.6 A^2 |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 341 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
References
- "zucapsaicin (CHEBI:135952)". www.ebi.ac.uk. Retrieved 2019-06-25.
- "Zucapsaicin". www.drugbank.ca. Retrieved 2019-06-25.
- Studer, Milena; McNaughton, Peter A. (2010-10-01). "Modulation of single-channel properties of TRPV1 by phosphorylation: Modulation of TRPV1 single channels by phosphorylation". The Journal of Physiology. 588 (19): 3743–3756. doi:10.1113/jphysiol.2010.190611. PMC 2998224. PMID 20693293.
- Winston Pharmaceuticals website "Archived copy". Archived from the original on April 25, 2012. Retrieved November 16, 2011.CS1 maint: archived copy as title (link)
- Sałat, Kinga; Jakubowska, Anna; Kulig, Katarzyna (October 2014). "Zucapsaicin for the treatment of neuropathic pain". Expert Opinion on Investigational Drugs. 23 (10): 1433–1440. doi:10.1517/13543784.2014.956079. ISSN 1354-3784. PMID 25171227.
- Zucapsaicin information from the National Library of Medicine http://druginfo.nlm.nih.gov/drugportal
- Bevan SJ, Docherty RJ. Cellular mechanisms of the action of capsaicin. In: Wood J, editor. Capsaicin in the study of pain. Academic Press, London, England; 1993. p. 27-44
- Anand, P.; Bley, K. (October 2011). "Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch". British Journal of Anaesthesia. 107 (4): 490–502. doi:10.1093/bja/aer260. ISSN 0007-0912. PMC 3169333. PMID 21852280.
- St. Pierre, Michael; Reeh, Peter W.; Zimmermann, Katharina (2009-04-30). "Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro". Experimental Brain Research. 196 (1): 31–44. doi:10.1007/s00221-009-1808-3. ISSN 0014-4819. PMID 19404626.
- Stucky CL, Dubin AE, Jeske NA, et al. Roles of transient receptor potential channels in pain. Brain Res Rev 2009;60(1):2-23
- Nilius, B.; Owsianik, G. (2013). "Transient Receptor Potential Family of Ion Channels". Encyclopedia of Pain. Genome Biology. 12. Springer Berlin Heidelberg. p. 4037. doi:10.1007/978-3-642-28753-4_202324. ISBN 9783642287527. PMC 3129667. PMID 21401968.
- Szallasi, Arpad; Sheta, Mohamed (2012-07-11). "Targeting TRPV1 for pain relief: limits, losers and laurels". Expert Opinion on Investigational Drugs. 21 (9): 1351–1369. doi:10.1517/13543784.2012.704021. ISSN 1354-3784. PMID 22780443.
- Trevisani, Marcello (2010-07-26). "Targeting TRPV1: Challenges and Issues in Pain Management~!2009-12-02~!2010-03-08~!2010-07-26~!". The Open Drug Discovery Journal. 2 (3): 37–49. doi:10.2174/1877381801002030037. ISSN 1877-3818.
- Khairatkar-Joshi, Neelima; Szallasi, Arpad (January 2009). "TRPV1 antagonists: the challenges for therapeutic targeting". Trends in Molecular Medicine. 15 (1): 14–22. doi:10.1016/j.molmed.2008.11.004. ISSN 1471-4914. PMID 19097938.
- Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol 2013;716:61-76
- Papoiu, Alexandru DP; Yosipovitch, Gil (2010-05-06). "Topical capsaicin. The fire of a 'hot' medicine is reignited". Expert Opinion on Pharmacotherapy. 11 (8): 1359–1371. doi:10.1517/14656566.2010.481670. ISSN 1465-6566. PMID 20446852.
- Palazzo, Enza; Luongo, Livio; de Novellis, Vito; Berrino, Liberato; Rossi, Francesco; Maione, Sabatino (January 2010). "Moving towards Supraspinal TRPV1 Receptors for Chronic Pain Relief". Molecular Pain. 6: 1744–8069-6-66. doi:10.1186/1744-8069-6-66. ISSN 1744-8069. PMC 2959024. PMID 20937102.
- Palazzo, Enza Luongo, Livio de Novellis, Vito Berrino, Liberato Rossi, Francesco Maione, Sabatino (2010-10-11). Moving towards supraspinal TRPV1 receptors for chronic pain relief. BioMed Central Ltd. OCLC 754846779.CS1 maint: multiple names: authors list (link)
- Lambert, D.G. (February 2009). "Capsaicin receptor antagonists: a promising new addition to the pain clinic". British Journal of Anaesthesia. 102 (2): 153–155. doi:10.1093/bja/aen354. ISSN 0007-0912. PMID 19151045.
- Lambert, G. A.; Davis, J. B.; Appleby, J. M.; Chizh, B. A.; Hoskin, K. L.; Zagami, A. S. (2009-08-19). "The effects of the TRPV1 receptor antagonist SB-705498 on trigeminovascular sensitisation and neurotransmission". Naunyn-Schmiedeberg's Archives of Pharmacology. 380 (4): 311–325. doi:10.1007/s00210-009-0437-5. ISSN 0028-1298. PMID 19690836.
- Holzer, P. (March 1988). "Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides". Neuroscience. 24 (3): 739–768. doi:10.1016/0306-4522(88)90064-4. ISSN 0306-4522. PMID 3288903.
- 1. Sanofi Canada: ZUACTA (zucapsaicin cream) product monograph [Link]
- Bernstein JE. Method and compositions of civamide to treat disease of the intestines. WO2011100668 A4; 2011
- Schnitzer TJ, Pelletier JP, Haselwood DM. Civamide cream 0.075% in patients with osteoarthritis of the knee: a 12-week randomized controlled clinical trial with a longterm extension. J Rheumatol 2012;39(3):610-20
- "Zucapsaicin. Computed Properties". pubchem.ncbi.nlm.nih.gov. Retrieved August 23, 2019.