APINACA
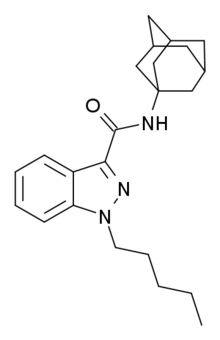
APINACA (AKB48, N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors,[1] with a Ki of 304.5nM and an EC50 of 585nM at CB1. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends, along with a related compound APICA.[2] Structurally, it closely resembles cannabinoid compounds from a University of Connecticut patent (WO 2003/035005),[3] but with a simple pentyl chain on the indazole 1-position, and APINACA falls within the claims of this patent despite not being disclosed as an example.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H31N3O |
| Molar mass | 365.521 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Legality
APINACA was made illegal in Japan in 2012,[4] and was banned as a temporary class drug in New Zealand from 13 July 2012.[5]
APINACA has been banned in Latvia since 14 November 2013.
Since 2013, APINACA has been a Schedule I controlled substance in the United States.[6]
It is also banned in Germany as an Anlage II controlled drug.
APINACA is listed in the Fifth Schedule of the Misuse of Drugs Act (MDA) and therefore illegal in Singapore as of May 2015.[7]
As of October 2015 APINACA is a controlled substance in China.[8]
APINACA is banned in the Czech Republic.[9]
Detection
A forensic standard of APINACA is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[10]
See also
References
- Uchiyama, N.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. (2012). "URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products". Forensic Science International. 227 (1–3): 21–32. doi:10.1016/j.forsciint.2012.08.047. PMID 23063179.
- Uchiyama, N.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. (2012). "Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products". Forensic Toxicology. 30 (2): 114. doi:10.1007/s11419-012-0136-7.
- WO 2003/035005
- "Designation of "Shitei Yakubutsu" (designated substances) based on the provision of the Pharmaceutical Affairs Law (1960, Law No.145)" (PDF).
- "Temporary Class Drug Notice". Department of Internal Affairs. New Zealand. 5 July 2012.
- "DEA Makes Three More "Fake Pot" Drugs Temporarily Illegal Today". Drug Enforcement Administration (DEA). Retrieved 24 July 2015.
- "CNB NEWS RELEASE". Central Narcotics Bureau (CNB). 30 April 2015. Archived from the original on 15 July 2015. Retrieved 24 July 2015.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.
- "APINACA". Southern Association of Forensic Scientists. Archived from the original on 24 July 2015. Retrieved 24 July 2015.