Synthetic cannabinoids
Synthetic cannabinoids are a class of molecules that bind to the same receptors to which cannabinoids in cannabis plants THC and CBD attach. They are designer drugs, commonly sprayed onto plant matter[1] and are usually smoked,[2] although they have also been consumed in a concentrated liquid form in the US and UK since 2016.[3] They have been marketed as herbal incense, or “herbal smoking blends”[2] and sold under common names like K2, Spice,[4] and Synthetic Marijuana.[1] They are often labeled “not for human consumption” for liability defense.[4] A large and complex variety of synthetic cannabinoids are designed in an attempt to avoid legal restrictions on cannabis, making synthetic cannabinoids designer drugs.[2]

Most synthetic cannabinoids are agonists of the cannabinoid receptors. They have been designed to be similar to THC,[5] the natural cannabinoid with the strongest binding affinity to the CB1 receptor, which is linked to the psychoactive effects or "high" of marijuana.[6] These synthetic analogs often have greater binding affinity and greater potency to the CB1 receptors. There are several synthetic cannabinoid families (e.g. CP-xxx, WIN-xxx, JWH-xxx, UR-xxx, and PB-xx) classified based on the base structure.[7]
When the herbal blends went on sale in the early 2000s, it was thought that they achieved psychoactive effects from a mixture of natural herbs. Laboratory analysis in 2008 showed instead that many contained synthetic cannabinoids.[2] Since 2016, synthetic cannabinoids are the most common new psychoactive substances to be reported. From 2008 to 2014, 142 synthetic cannabinoids were reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA).[8]
Reported user negative effects include palpitations, paranoia, intense anxiety, nausea, vomiting, confusion, poor coordination, and seizures. There have also been reports of a strong compulsion to re-dose, withdrawal symptoms, and persistent cravings.[8] There have been several deaths linked to synthetic cannabinoids. The Centers for Disease Control and Prevention (CDC) found that the number of deaths from synthetic cannabinoid use tripled between 2014 and 2015.[9][10]In 2018, the United States Food and Drug Administration warned of significant health risks from synthetic cannabinoid products that contain the rat poison brodifacoum, which is added because it is thought to extend the duration of the drugs' effects.[11] Severe illnesses and death have resulted from this contamination.[11]
Contents of synthetic cannabinoid blends
Just as the synthetic cannabinoid(s) used differ between each synthetic cannabinoid product sold, so do the other contents of the “herbal blends.” Packages of synthetic cannabinoid products can claim to contain a wide array of plants including alfalfa, blue violet, nettle leaf, marsh mallow, rosehip, white or blue water lily, honeyweed, dwarf skullcap, and others. Oftentimes, none of the listed ingredients have been detectable. It is often difficult to determine what is in these products because masking agents, such as tocopherol (vitamin E), eugenol, and fatty acids, are added to confound identification. Most products consist of synthetic cannabinoids sprayed onto inert vegetable matter, but some contain other psychoactive substances, including psychoactive herbs, e.g., “Wild Dagga” and “Indian Warrior,” and psychoactive alkaloids, e.g., betonicine, aporphine, leonurine, nuciferine, and nicotine. Some synthetic cannabinoids products have also been found to contain synthetic opioids including O-desmethyltramadol and AH-7921. In 2010, nine people died due to the combination of O-desmethyltramadol, a μ-opioid agonist and analgesic drug, and Kratom, an Asiatic medicinal plant containing mitragynine, another μ-opioid agonist, in a synthetic cannabinoid product called “Krypton.”[12] In 2013, AH-7921 was detected in smoking blends in Japan.[13]
One of the most common non-cannabinoid ingredients in these products is oleamide, a fatty acid derivative that acts similarly to a cannabinoid and has hypnotic properties. Other non-cannabinoid ingredients that have been found in synthetic cannabinoid blends include harmine and harmaline, reversible monoamine oxidase inhibitors, which have been found with myristicin and asarone;[12] substituted cathinone derived stimulant drugs such as 4-methylbuphedrone and 4'-methyl-alpha-PPP; and psychedelic tryptamine derivatives such as 4-OH-DET.[14][15]
Naming synthetic cannabinoids
Many of the early synthetic cannabinoids that were synthesized for use in research were named after either the scientist who first synthesized them or the institution or company where they originated. For example, JWH compounds are named after John W. Huffman and AM compounds are named after Alexandros Makriyannis, the scientists who first synthesized those cannabinoids. HU compounds are named after Hebrew University in Jerusalem, the institution where they were first synthesized, and CP compounds are named after Carl Pfizer, the company where they were first synthesized.
Some of the names of synthetic cannabinoids synthesized for recreational use were given names to help market the products. For example, AKB-48 is also the name of a popular Japanese girl band; 2NE1 is also a South Korean girl band; and XLR-11 was named after the first USA-developed liquid fuel rocket for aircraft. Now many synthetic cannabinoids are assigned names derived from their chemical names. For example, APICA (also known as 2NE1) comes from N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide and APINACA (also known as AKB-48) comes from N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide.[16]
Common names
Use of the term “synthetic marijuana” to describe products containing synthetic cannabinoids is controversial and, according to Dr. Lewis Nelson, a medical toxicologist at the NYU School of Medicine, a misnomer. Nelson claims that relative to marijuana, products containing synthetic cannabinoids “are really quite different, and the effects are much more unpredictable. It’s dangerous.”[17] Since the term synthetic does not apply to the plant, but rather to the cannabinoid that the plant contains (THC), the term synthetic cannabinoid is more appropriate.[18]
Synthetic cannabinoids are known by a number of brand names including K2, Spice, Arizona, Black Mamba, Bombay Blue, Genie, Zohai,[19] Banana Cream Nuke, Krypton, Lava Red, and many more.[12] In some Spanish-speaking countries, such as Chile and Argentina, such preparations are often referred to as "cripy". They are often called “synthetic marijuana”, “natural herbs”, “herbal incense”, or “herbal smoking blends” and often labeled “not for human consumption”.[4] They are increasingly offered in e-cigarette form as "c-liquid"[20] with brand names such as Kronic.[21]
According to the Psychonaut Web Mapping Research Project, synthetic cannabinoids, sold under the brand name “Spice”, were first released in 2005 by the now-dormant company The Psyche Deli in London, UK. In 2006, the brand gained popularity. According to the Financial Times, the assets of The Psyche Deli rose from £65,000 in 2006 to £899,000 in 2007. The EMCDDA reported in 2009 that Spice products were identified in 21 of the 30 participating countries.[22]
Uses
Synthetic cannabinoids were made for cannabinoid research focusing on tetrahydrocannabinol (THC), the main psychoactive and analgesic compound found in the cannabis plant. Synthetic cannabinoids were needed partly due to legal restrictions on natural cannabinoids, which make them difficult to obtain for research. Tritium-labelled cannabinoids such as CP-55,940 were instrumental in discovering the cannabinoid receptors in the early 1990s.[23]
Some early synthetic cannabinoids were also used clinically. Nabilone, a first generation synthetic THC analog, has been used as an antiemetic to combat vomiting and nausea since 1981. Synthetic THC (marinol, dronabinol) has been used as an antiemetic since 1985, and an appetite stimulant since 1991.[24]
In the early 2000s, synthetic cannabinoids began to be used for recreational drug use in an attempt to get similar effects to cannabis. Because synthetic cannabinoid molecular structures differ from THC and other illegal cannabinoids, synthetic cannabinoids were not technically illegal. Since the discovery of the use of synthetic cannabinoids for recreational use in 2008, some synthetic cannabinoids have been made illegal, but new analogs are continually synthesized to avoid the restrictions. Synthetic cannabinoids have also been used recreationally because they are inexpensive and are typically not revealed by the standard marijuana drug tests. Unlike nabilone, the synthetic cannabinoids found being used for recreational use did not have any documented therapeutic effects.[12]
Toxicity
There are no fatal overdose cases linked to marijuana,[25] but deaths associated with synthetic cannabinoids are increasing.[26] The CDC found that the number of deaths from synthetic cannabinoid use tripled between 2014 and 2015.[10] These drugs are dangerous because they are more potent than marijuana, and due to the large quantity of different structures that fall under the same common names, users are often unaware of exactly what they are getting and how potent it is.[27] For example, Δ9-THC has an EC50 of 250 nM at CB1 and 1157 nM at CB2, whereas PB-22 has an EC50 of 5.1 nM at CB1 and 37 nM at CB2.[4]
No official studies have been conducted on the effects of synthetic cannabinoids on humans (as is often the case with illegal and potentially toxic compounds);[28] however, user reports and the effects experienced by patients seeking medical care after taking synthetic cannabinoids have been published. Each of the many different synthetic cannabinoids can have different effects, particularly different effects at different dosages. Some negative effects of 5F-PB-22 reported by users included nausea, vomiting, confusion, poor coordination, anxiety, and seizures. Some of the negative effects of 5F-AKB-48 reported by users included palpitations, paranoia, intense anxiety, and a taste like burned plastic.[8] The CDC recorded negative effects of synthetic cannabinoid overdose between 2010 and 2015 and of 277 drug overdose patients who reported synthetic cannabinoid as the sole agent, 66.1% reported problems in the central nervous system (e.g., agitation, coma, toxic psychosis), 17% reported cardiovascular problems (e.g., tachycardia, bradycardia), 7.6% reported pulmonary problems (5.4% of which had respiratory depression), and 4% reported acute kidney injury.[29]
There have also been reports of a strong compulsion to re-dose, withdrawal symptoms, and persistent cravings lasting up to a week after taking synthetic cannabinoids, indicating that synthetic cannabinoids may be significantly more addictive than marijuana.[8] The greater addictiveness and more severe adverse effects of synthetic cannabinoids in comparison to marijuana are thought to stem from the fact that many of the synthetic cannabinoids are full agonists to the cannabinoids receptors, CB1 and CB2, compared to THC, which is only a partial agonist.[30] It has also been seen that phase 1 metabolism of JWH-018 results in at least nine monohydroxylated metabolites, three of which have been shown to be full agonists of the CB1 receptors, compared to the metabolism of THC, which only results in one psychoactive monohydroxylated metabolite. This may further explain the increased toxicity of synthetic cannabinoids compared to THC.[31]
Four postmortem cases linked to the synthetic cannabinoids 5F-PB-22 have been reviewed. The postmortem blood specimens contained a range of 1.1-1.5 ng/mL of 5F-PB-22. Three of the four cases were sudden episodes and the symptoms leading to death included acute shortness of breath; vasocongestion in the liver, spleen, and kidneys; bilateral pulmonary edema; dead inflamed tissue (necrotizing granulomatous inflammation); and congestion of most internal organs. The fourth case presented to the hospital with severe problems that deteriorated over the course of a day, ending with circulatory, respiratory, central nervous system, and renal failure.[32]
Psychosis
Studies are currently available that suggest an association between synthetic cannabinoids and psychosis.[33] The use of synthetic cannabinoids can be associated with psychosis and physicians are beginning to investigate if some patients with inexplicable psychotic symptoms may have at one point used synthetic cannabinoids. In contrast to most other recreational drugs, the dramatic psychotic state induced by use of synthetic cannabinoids has been reported, in multiple cases, to persist for several weeks, and in one case for seven months, after complete cessation of drug use.[34] Some studies suggest that not only can synthetic cannabinoids induce psychosis, but they can worsen previously stable psychotic disorders and might trigger a chronic (long-term) psychotic disorder among vulnerable individuals such as those with a family history of mental illness.[35] Individuals with risk factors for psychotic disorders are often counseled against using synthetic cannabinoids.[36] Psychiatrists have suggested that the lack of an antipsychotic chemical, like CBD in natural cannabis, may make synthetic cannabinoids more likely to induce psychosis than natural cannabis.[37]
Stroke
Two patients experienced difficulty speaking and weakness across one half of their body shortly after smoking Spice. CT angiography confirmed both had experienced an ischemic stroke[38]
Structural classifications
| Classification | Examples | |
|---|---|---|
| 1 | Naphthoylindoles
Naphthylmethylindoles Naphthoylpyrroles Naphthylmethylindenes |
JWH-007, JWH-018,
JWH-398, AM-1221, AM-2201, AM-694, WIN-55,212-2 |
| 2 | Phenylacetylindoles
(i.e., benzoylindoles) |
JWH-250, RCS-8 |
| 3 | Cyclohexylphenols | CP-47,947, CP-55,940 |
| 4 | Tetramethylcyclopropylindoles | UR-144, XLR-11 |
| 5 | Adamantoylindoles | 5F-AKB-48, APICA, STS-135 |
| 6 | Indazole carboxamides | AB-PINACA, AB-FUBINACA |
| 7 | Quinolinyl ester | PB-22, 5F-PB-22 |
There are five major categories for synthetic cannabinoids: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids, aminoalkylindoles, and eicosanoids. Classical cannabinoids are analogs of THC that are based on a dibenzopyran ring. They were developed starting in the 1960s, following the isolation of THC,[22] and were originally the only cannabinoids synthesized.[40] Classical cannabinoids include nabilone and dronabinol, and one of the best known synthetic classical cannabinoids is HU-210.[41] HU-210 is a chiral compound first synthesized by Raphael Mechoulam at Hebrew University in the 1980s. It was discovered in herbal incense products by the U.S. Customs and Border Protection in January 2009; however, classical cannabinoids are not often seen in synthetic cannabinoid blends for recreational use, likely because they are difficult to synthesize.[42]
Non-classical cannabinoids include cyclohexylphenols (CP), which were first synthesized in the late 1970s to 1980s by Pfizer as potential analgesics.[41] The C8 homologue of CP-47,497 (CP-47,497-C8) was one of the first synthetic cannabinoids being used recreationally. CP-47,497-C8 is made by extending the dimethylheptyl side chain of CP-47,497 to a dimethyloctyl side chain. It was discovered by forensic scientists in an herbal blend known as “Spice” in 2008, along with JWH-018, an aminoalkylindole.[4]
Hybrid cannabinoids have a combination of classical and non-classical cannabinoid structural features.[40] For example, AM-4030, a derivative of HU-210, is a hybrid cannabinoid because it has the dibenzopyran ring common of classical cannabinoids and an aliphatic hydroxyl group common in the CP family of nonclassical cannabinoids.[43]
Aminoalkylindoles are structurally dissimilar to THC and include naphthoylindoles (JWH-018), phenylacetylindoles (JWH-250), and benzoylindoles (AM-2233). Aminoalkylindoles are considered to be the most common synthetic cannabinoids found in synthetic cannabinoid blends, likely due to the fact that these molecules are easier to synthesize than classical and non-classical cannabinoids. The JWH molecules were first synthesized by Professor John William Huffman at Clemson University in the late 1990s.[41] The FBI concluded in a 2012 memo that as a result of the publication of J.W. Huffman’s research, people searching for a “marijuana-like-high” would follow his recipes and methods.[1]
Eicosanoid synthetic cannabinoids are analogs of endocannabinoids, such as anandamide. Endocannabinoids are cannabinoids naturally occurring in the body. One of the best known synthetic analogs of anandamide is methanandamide.[40]
The synthetic cannabinoids that have emerged recently have even greater structural diversity, possibly to subvert legal regulations on earlier generations of synthetic cannabinoids. There are a few different structural classifications of synthetic cannabinoids that include many of the new structures, some of which are shown in Table 1. The indazole carboxamide group, including APINACA (AKB-48), an adamantyl indazole carboxamide, and AB-PINACA, an aminocarbonyl indazole carboxamide, is an example of a new group of synthetic cannabinoids.[41] Most clandestine manufacturers and producers only make small changes to the structure of a synthetic cannabinoid, such as changing an indole to indazole structure (AM-2201 to THJ-2201) or terminal fluorine replacement;[3] however, one group that was unprecedented when discovered by forensic scientists in 2013, was the quinolinyl ester synthetic cannabinoids.[4]
PB-22 and 5F-PB-22 were the first synthetic cannabinoids to include a quinoline substructure and an ester linkage. These compounds are thought to have been synthesized with the intention of making a synthetic cannabinoid prodrug, which might improve absorption and confound detection. Ester bonds are easily biodegradable through spontaneous or endogenous, nonspecific esterase hydrolysis, which has been commonly used in medicinal chemistry to make ester prodrugs. The reason for the change to the quinolone substructure is unknown, but it may have been found to be a suitable replacement for the naphthoyl moiety that is currently regulated by US scheduling laws.[39]
Although most synthetic cannabinoids are not direct analogs of THC, they share many common features with THC. Most are lipid-soluble, non-polar, small molecules (usually 20-26 carbon atoms) that are fairly volatile, making them “smokable,” like THC.[22] Another common feature of most synthetic cannabinoids and THC is a side-chain of 5-9 saturated carbon atoms. It has been found that this chain of 5-9 carbons is required for optimal psychotropic activity from binding CB1 receptors.[12] Also, most synthetic cannabinoids are agonists of both cannabinoid receptors, CB1 and CB2, like THC; however, they often have greater binding affinity and therefore greater potency than THC, as seen in Table 2. Due to the greater potency, the standard doses of many synthetic cannabinoids may be less than 1 mg.[22]
| Name | Year Identified by Forensics | Structural Classification | Structure | CB1 Binding Affinity (nM)[44] | CB2 Binding Affinity (nM)[44] | CB1 EC50 (nM)[4] | CB2 EC50 (nM)[4] |
|---|---|---|---|---|---|---|---|
| Δ9-THC | Classical Cannabinoid | 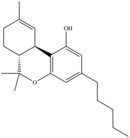 |
41 ± 2 | 36 ± 10 | 250 | 1157 | |
| HU-210 | 2009[12] | Classical Cannabinoid | 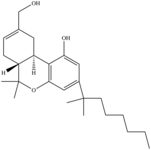 |
0.061 ± 0.007 | 0.52 ± 0.04 | ||
| (C8) CP 47,497 | 2008[4] | Non-Classical Cannabinoid (Cyclohexylphenol) | .png) |
2.20 ± 0.47 | |||
| JWH-018 | 2008[4] | Aminoalkylindole (Naphthoylindoles) | 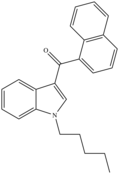 |
9.0 ± 5.0[4] | 2.94 ± 2.65[4] | 102 | 133 |
| AM-2201 (Fluorinated JWH-018) | 2011[4] | Aminoalkylindole (Naphthoylindoles) | 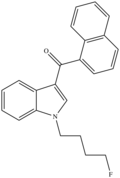 |
1.0 | 2.6 | 38 | 58 |
| UR-144 | 2010[4] | Tetramethylcyclopropylindoles | 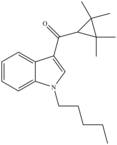 |
29 ± 0.9 | 4.5 ± 1.7 | 421 | 72 |
| XLR-11 (Fluorinated UR-144) | 2012[4] | Tetramethylcyclopropylindoles | 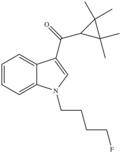 |
24 ± 4.6 | 2.1 ± 0.6 | 98 | 83 |
| APICA | 2012 | Adamantoylindole | 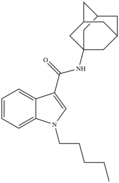 |
128[45] | 29[45] | ||
| STS-135 (Fluorinated APICA) | Adamantoylindole | 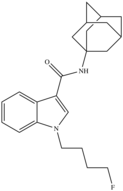 |
51 | 13 | |||
| AB-PINACA | 2012[46] | Indazole carboxamide | 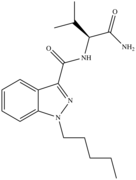 |
1.2 | 2.5 | ||
| PB-22 | 2013[4] | Quinolinyl ester | 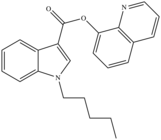 |
5.1 | 37 | ||
| 5F-PB-22 (Fluorinated PB-22) | Quinolinyl ester | 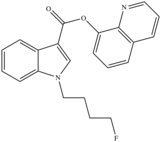 |
0.468[47] | 0.633[47] | 2.8 | 11 |
Stereospecificity
Most classical, non-classical, and hybrid synthetic cannabinoids have stereospecificity (one stereoisomer is usually much more potent than the other(s)). For example, HU-210 is the (–) enantiomer of 11-OH-Δ8-THC-DMH and a full agonist of the CB1 receptor;[48] the (+) enantiomer of 11-OH-D8-THC-DMH, known as HU-211, is a NMDA receptor antagonist and is largely inactive as a cannabinoid.[49] On the other hand, aminoalkylindoles, eicosanoids, and the other new synthetic cannabinoid groups typically do not have an asymmetric center, so they are usually not stereospecific.[40]
Fluorination of terminal carbon
Recently there has been an increase in the emergence of terminally fluorinated synthetic cannabinoids, such as 5F-PB-22 (fluorinated version of PB-22) and XLR-11 (fluorinated version of UR-144). South Korea’s National Forensic Service reported that 90% of all seized synthetic cannabinoids in 2013 were fluorinated, compared to no fluorinated synthetic cannabinoids reported in 2010. 5F-derivations (terminal fluorination) of the synthetic cannabinoids have been found to be about 2-5 times more potent at CB1 receptors than their un-fluorinated counterparts,[4] as shown in Table 2.
Detection in bodily fluids
Synthetic cannabinoids are typically not identified by the standard marijuana drug tests including the immunoassay test (EMIT), GC-MS screening, and multi-target screening by LC-GC/MS because those tests only detect the presence of THC and its metabolites.[50][51] Although most synthetic cannabinoids are analogs of THC, they are structurally different enough that, for example, the specific antibodies in the EMIT for marijuana do not bind to them.[52] Also, due to their high potency, a very small dose of synthetic cannabinoids is used; moreover, synthetic cannabinoids are highly metabolized by the body, so the window to detect the parent drug (the synthetic cannabinoid itself) in blood and oral fluid is very small.[53]
Serum concentrations of synthetic cannabinoids are generally in the 1–10 μg/L range during the first few hours after recreational usage and the metabolites are usually present in urine at similar concentrations.[54] Little to no parent drug is present in urine, so there is a lot of research to try and identify the major urinary metabolites that could be used as markers of synthetic cannabinoid intake.[3] The major urinary metabolites in most cases are formed by oxidation of the alkyl side-chain to an alcohol and carboxylic acid followed by glucuronide conjugation and also by N-dealkylation and aromatic hydroxylation.[55] For example, the main metabolites of JWH-018, of which there are over 20, include carboxylated, monohydroxylated, dihydroxylated, and trihydroxylated metabolites, but they are mostly excreted in urine as glucuronide conjugates.[42] The presence of synthetic cannabinoids or their metabolites in bodily fluids may be determined using specifically-targeted commercially available immunoassay screening methods (EMIT), while liquid chromatography-mass spectrometry is most often used for confirmation and quantitation.[56][57][58] There are commercially available EMIT kits for the screening of the synthetic cannabinoids JWH-018, JWH-073, JWH-398, JWH-200, JWH-019, JWH-122, JWH-081, JWH-250, JWH-203, CP-47,497, CP-47,497-C8, HU-210, HU-211, AM-2201, AM-694, RCS-4, and RCS-8 through companies like NMS Labs, Cayman Chemical, and Immunoanalysis Corporation.[59]
Notable Incidents
On October 20, 2011, the Louisiana State University football program announced that it had suspended three players, including star cornerback Tyrann Mathieu, who tested positive for synthetic cannabinoids.[60]
In the autumn of 2014, more than two-thousand Spice consumers in Russia sought medical attention, one-thousand were admitted to hospitals, and 40 people died.[61]
On July 12, 2016, 33 people were intoxicated by an herbal “incense” product called “AK-47 24 Karat Gold,”[62] and dozens overdosed, in Brooklyn. 18 people were transported to local hospitals.[63] This mass intoxication event was referred to as a “zombie” outbreak in the press due to the description of the intoxicated persons by bystanders as “zombielike” (moved slowly, had blank stares, and occasionally groaned).[64] The herbal “incense” product was determined to be a synthetic cannabinoid called AMB-FUBINACA.[62]
Since March 2018, Illinois, Wisconsin, Maryland, and 8 other states in the United States have had an outbreak of severe bleeding caused by a synthetic cannabinoid contaminated with brodifacoum, a rat poison that causes bleeding. Illinois was hit the hardest[65] and on April 5, 2018, the CDC issued a Clinical Action alert to health care providers across the United States advising of 89 confirmed cases of “serious unexplained bleeding” in Illinois. The cases are still being studied; however, 63 of the patients reported synthetic cannabinoid use, and laboratory analysis confirmed brodifacoum was present in at least 18 patients.[66] As of April 24, 2018, 153 cases, including four deaths, linked to this outbreak have been reported to the Illinois Department of Public Health (IDPH) since March 7, 2018.[67] On September 18, 2018, the Wisconsin Department of Health Services confirmed 16 more cases, bringing the total number of people affected by the outbreak in Wisconsin to 80 people since March 2018, including one death in July 2018.[68]
In August 2018, there were almost one hundred overdose cases reported over two days in New Haven, Connecticut from a bad batch of K2. The synthetic cannabinoid was believed to have been mixed with fentanyl, although no fentanyl was identified in samples of the drug tested by the DEA.[69]
In September 2018, at least 10 people overdosed on a synthetic cannabinoid, either AMB-FUBINACA or AB, in Christchurch, New Zealand over two days. Some of the people are in critical condition in the Intensive Care Unit.[70]
From September 21–22, 2018, almost 50 people overdosed and two people died in the Kensington area of Philadelphia. Tests are still in progress, but officials believe the cause to be a combination of heroin or fentanyl and a synthetic cannabinoid.[71] This same area in Philadelphia had 155 people overdose and 10 people die from a combination of heroin, fentanyl, and a synthetic cannabinoid called 5F-ADB over one weekend in July 2018. The Department of Public Health released that they believe "5F-ADB was the primary cause of the cluster of patients with these adverse drug reactions."[72]
Legal restrictions and regional availability
Europe
Austria
The Austrian Ministry of Health announced on December 18, 2008 that Spice would be controlled under Paragraph 78 of their drug law on the grounds that it contains an active substance that affects the functions of the body, and the legality of JWH-018 is under review.[73][74][75]
Germany
JWH-018, CP 47,497 and the C6, C8 and C9 homologues of CP 47,497 have been illegal in Germany since January 22, 2009.[76][77] Since November 26, 2016 about 80-90% of the substances belonging to the group of synthetic cannabinoids are illegal in Germany as the law does not cover all chemical structures.[78]
France
JWH-018, CP 47,497 (and its homologues), and HU-210 were all made illegal in France on February 24, 2009.[79]
Ireland
From June 2010, JWH-018, along with a variety of other designer drugs, has been illegal.[80]
Latvia
JWH-018, JWH-073, CP 47,497 (and its homologues), and HU-210 as well as leonotis leonurus have been all banned in Latvia since 2005.[81] After the first confirmed lethal case from the use of legal drugs in late 2013, parliament significantly increased the number of temporarily banned substances used in Spice and similar preparations. On April 3, 2014, parliament made selling of the temporarily banned substances a criminal offense.[82]
Poland
JWH-018 and many of the herbs mentioned on the ingredient lists of Spice and similar preparations were made illegal in May 2009. The bill was passed by Polish Sejm[83][84] and Polish Senat[85] and was signed by the President.[86]
Romania
Spice was made illegal in Romania on February 15, 2010. As on 12 September 2018 Spice was made legal for personal use.[87]
Russia
On April 9, 2009, the Chief Medical Officer of the Russian Federation issued a resolution on reinforcing control over the sales of smoking blends. These blends, marketed under the trade names AM-HI-CO, Dream, Spice (Gold, Diamond), Zoom, Ex-ses, Yucatán Fire and others, have been declared to contain Salvia divinorum, Hawaiian wood rose, and blue lotus, and are prohibited to be sold. These substances have been found to have "psychotropic, narcotic effects, contain poisonous components and represent potential threat for humans". The resolution does not mention JWH-018 or other synthetic cannabinoids.[88] On January 14, 2010, the Russian government issued a statement including 23 synthetic cannabinoids found in smoking blends Hawaiian Rose and Blue Lotus on the list of prohibited narcotic and psychotropic substances.[89]
About 780 new psychoactive substances were added to the list from 2011 to 2014. The drugmakers avoided all the bans by making slight changes to the drugs. In the autumn of 2014, more than two-thousand Spice consumers in Russia sought medical attention, one-thousand were admitted to hospitals, and 40 people died[61] On October 30, 2014, President Vladimir Putin brought in a bill that increased the penalty for selling or consuming smoking blends from a fine to up to eight years in prison.[90]
Slovakia
Spice is legal in Slovakia. The National Anti-Drug Unit is considering adding it to the list of controlled substances.[91] The latest anti-drug law version (468/2009) valid since January 2010 does not mention active compounds of Spice.[92]
Spain
Spice is unregulated in Spain. For this reason, Spice is available in grow shop stores or cannabis related stores, and it can be bought and shipped online without any legal impediment from those kind of stores.[93] However, as Spanish law permits growing up to two cannabis plants per household, and the possession and consumption of cannabis on private property is also legal, cannabis alternatives like Spice are largely redundant and thus remain relatively unknown in Spain.[94]
Sweden
CP 47,497-C6, CP 47,497-C7, CP 47,497-C8, CP 47,497-C9, JWH-018, JWH-073 and HU-210 were all made illegal in Sweden on September 15, 2009. The bill was accepted on July 30, 2009 and was put in effect on September 15, 2009.[95]
Switzerland
Spice has been banned in Switzerland.[96]
Turkey
Spice, which is colloquially called bonzai in Turkey, was added to the list of drugs and psychotropic substances on July 1, 2011 by the law numbered as 2011/1310 B.K.K. (February 13, 2011 and the Official Gazette No. 27845).[97]
United Kingdom
Many cannabinoids were legal in the United Kingdom until December 2009, when they were classified as Class B drugs.[98] The UK continued to ban new synthetic cannabinoids as they came to market but they were typically replaced instantly by novel alternatives. In May 2016, the Psychoactive Substances Act[99] came into force, which intends to restrict the production, sale and supply of new psychoactive substances.
North America
Canada
Spice is not specifically prohibited in Canada, but synthetic cannabis mimics are listed as a schedule II drug. Schedule II to the Controlled Drugs and Substances Act makes reference to specific synthetic compounds JWH-XXX and AM-XXXX, although is not limiting to those identified.[100][101] Health Canada is debating the subject.[102][103] Schedule II has consisted entirely of synthetic cannabinoids since October 2018; these remain illegal following the removal from the schedule of cannabis and its constituents derived from nature.
United States
The case of David Mitchell Rozga, an American teenager from Indianola, Iowa, brought international attention to K2. Rozga shot himself in the head with a family-owned hunting rifle in an apparent suicide on June 6, 2010. After news of Rozga's death, it was reported by friends that they had smoked K2 with Rozga approximately one hour before his death. The nature of his death and reports from numerous family members, led investigators to suspect that Rozga was under the influence of a mind-altering substance when he died. The death of Rozga influenced political lobbying against K2, and other legal synthetic drugs such as bath salts. Following the incident, the "David Mitchell Rozga Act" to ban the use and distribution of K2 was introduced by Iowa Senator Chuck Grassley. It was passed by the United States Congress in June 2011.[104] On July 10, 2012, President Barack Obama signed the Synthetic Drug Abuse Prevention Act of 2012 into law. It banned synthetic compounds commonly found in synthetic marijuana, placing them under Schedule I of the Controlled Substances Act.[105]
Prior to that, some synthetic cannabis compounds (HU-210) were scheduled in the US under federal law, while others (JWH-073) were temporarily scheduled until final determination of their status could be made.[106][107][108][109] The Drug Enforcement Administration (DEA) considered K2 to be a "drug of concern",[110] citing "...a surge in emergency-room visits and calls to poison-control centers. Adverse health effects associated with its use include seizures, hallucinations, paranoid behavior, agitation, anxiety, nausea, vomiting, racing heartbeat, and elevated blood pressure."[111][112] Several states independently passed acts making it illegal under state law, including Kansas in March 2010,[113] Georgia and Alabama in May 2010,[114][115] Tennessee and Missouri in July 2010,[116][117] Louisiana in August 2010, Mississippi in September 2010, and Iowa.[118] An emergency order was passed in Arkansas in July 2010 banning the sale of synthetic cannabis mimics.[119] In October 2010, the Oregon Board of Pharmacy listed synthetic cannabinoid chemicals on its Schedule 1 of controlled substance, which means that the sale and possession of these substances is illegal under the Oregon Uniform Controlled Substances Act.[120] According to the National Conference of State Legislatures, several other states also considered legislation, including New Jersey, New York, Florida, and Ohio.[117] Illinois passed a law on July 27, 2010 banning all synthetic cannabinoids as of January 1, 2011.[121] Michigan banned synthetic cannabinoids in October 2010,[122] and the South Dakota Legislature passed a ban on these products which was signed into law by Gov. Dennis Daugaard on February 23, 2012 (and which took immediate effect under an emergency clause of the state constitution).[123] Indiana banned synthetic cannabinoids in a law which became effective in March 2012.[124] North Carolina banned synthetic cannabis mimics by a unanimous vote of the state senate, due to concerns that its contents and effects are reasonably similar to cannabis, and may cause equal effects in terms of psychological dependency.[125][126] Following cases in Japan involving the use of synthetic cannabinoids by navy, army and marine corps personnel, they were officially banned.[127] A punitive general order issued on January 4, 2010 by the Commander Marine Corps Forces, Pacific prohibits the actual or attempted possession, use, sale, distribution and manufacture of synthetic cannabis mimics as well as any derivative, analogue or variant of it.[128] On June 8, 2010, the US Air Force issued a memorandum that banned the possession and use of Spice, or any other mood-altering substance except alcohol or tobacco, among its service members.[129] On November 24, 2010, the DEA announced that it would make JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol, which are often found in synthetic cannabis mimics, illegal using emergency powers,[130] and placed them on Schedule I of the Controlled Substances Act.[131][132] The temporary ban, for at least a year, was effective as of March 1, 2011.[133]
South America
Chile
The Chilean Ministry of Health on April 24, 2009, declared the sale of synthetic cannabis mimics to be illegal.[134]
Asia
South Korea
South Korea officially added JWH-018, CP 47,497 and HU-210 to the controlled substance list on July 1, 2009, effectively making these chemicals illegal.[135]
Japan
Japan has banned JWH-018, CP 47, 497, and homologues, and HU-210 since October 2009.
United Arab Emirates
The United Arab Emirates had stated that Spice is an illegal substance and possession or intent to sell is a jailable offense.[136]
Australasia
Australia
On June 17, 2011, the Western Australian government banned all of the synthetic cannabinoids found in already existing products, including brands such as Kronic, Kalma, Voodoo, Kaos, and Mango Kush. Western Australia was the first state in Australia to prohibit the sale of certain synthetic cannabinoids.[137][21] On June 18, 2013, an interim ban made a large list of product brands and synthetic substances illegal to sell anywhere in Australia.[138] This ban lapsed on October 13, 2013, and a permanent ban has not been imposed.[139] Synthetic cannabinoids and related products remain illegal in NSW, where a bill was passed on September 18, 2013, that bans entire families of synthetic drugs instead of only banning existing compounds that have been identified.[140][141] The introduction of this law makes NSW the first state in Australia to completely ban substances with psychoactive properties.[141]
New Zealand
Spice is illegal in New Zealand, it is classified as a Class C controlled drug.[142] The New Zealand Parliament passed a law in July 2013 banning the sale of legal highs in dairies and supermarkets, but allowing some "low risk" drugs to continue to be sold through speciality licensed shops.[143] Synthetic cannabinoids, as well as all other legal highs were outlawed at midnight on 7 May 2014, after a law was passed a week prior by the New Zealand government.[144]
An analysis of 41 different synthetic cannabis mimic blends sold commercially in New Zealand, conducted by the Institute of Environmental Science and Research and released in July 2011, found 11 different synthetic cannabinoid ingredients used, including JWH-018, JWH-073, AM-694, AM-2201, RCS-4, RCS-4 butyl homologue, JWH-210, JWH-081, JWH-250 (or possibly JWH-302, isomer not determined), JWH-203, and JWH-122—with between one and five different active ingredients, though JWH-018 was present in 37 of the 41 blends tested. In two brands, the benzodiazepine anxiolytic drug phenazepam was also found, which is classified as a prescription medicine in New Zealand, and these brands were ordered to be removed from the market by emergency recall.[145][146] Since this time, a further 15 cannabinoid compounds have been detected as ingredients of synthetic cannabis mimicking blends in New Zealand and banned as temporary class drugs.[147] In 2013, another hypnotic medication, zaleplon, was found to have been used as an active ingredient in a blend that had been sold in New Zealand during 2011 and 2012.[148]
See also
- List of designer drugs § Synthetic cannabinoids
- Designer drug
- Cannabinoid receptor
- Cannabinoid
- Endocannabinoid enhancer
- Endocannabinoid system
- Structural scheduling of synthetic cannabinoids
- Tetrahydrocannabinol
References
- Macher, R.; Burke, T.W.; Owen, S.S. "Synthetic Marijuana". FBI: Law Enforcement Bulletin. Retrieved 2018-05-04.
- Science, Live (2017-02-07). "Synthetic Marijuana Linked To Seizures, Psychosis And Death". Huffington Post. Retrieved 2018-05-04.
- Diao, X; Huestis, MA (2016-11-24). "Approaches, Challenges, and Advances in Metabolism of New Synthetic Cannabinoids and Identification of Optimal Urinary Marker Metabolites". Clinical Pharmacology & Therapeutics. 101 (2): 239–253. doi:10.1002/cpt.534. ISSN 0009-9236. PMID 27727455.
- Banister, Samuel D.; Stuart, Jordyn; Kevin, Richard C.; Edington, Amelia; Longworth, Mitchell; Wilkinson, Shane M.; Beinat, Corinne; Buchanan, Alexandra S.; Hibbs, David E. (2015-05-08). "Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience. 6 (8): 1445–1458. doi:10.1021/acschemneuro.5b00107. ISSN 1948-7193. PMID 25921407.
- Cannaert, Annelies; Storme, Jolien; Franz, Florian; Auwärter, Volker; Stove, Christophe P. (2016-11-07). "Detection and Activity Profiling of Synthetic Cannabinoids and Their Metabolites with a Newly Developed Bioassay". Analytical Chemistry. 88 (23): 11476–11485. doi:10.1021/acs.analchem.6b02600. ISSN 0003-2700. PMID 27779402.
- Rapaka, R.S.; Makriyannis, A., eds. (1987). "Structure-Activity Relationships of the Cannabinoids" (PDF). NIDA Research Monograph. 79 – via U.S. Department of Health and Human Services.
- "K2: Scary Drug or Another Drug Scare?". Newsweek. 2010-03-03. Retrieved 2018-05-04.
- Abouchedid, Rachelle; Ho, James H.; Hudson, Simon; Dines, Alison; Archer, John R. H.; Wood, David M.; Dargan, Paul I. (2016-12-01). "Acute Toxicity Associated with Use of 5F-Derivations of Synthetic Cannabinoid Receptor Agonists with Analytical Confirmation". Journal of Medical Toxicology. 12 (4): 396–401. doi:10.1007/s13181-016-0571-7. ISSN 1556-9039. PMC 5135680. PMID 27456262.
- "Notes from the Field: Increase in Reported Adverse Health Effects Related to Synthetic Cannabinoid Use — United States, January–May 2015". www.cdc.gov. June 12, 2015. Retrieved 2018-11-01.
- "Frequently Asked Questions | Marijuana | CDC". www.cdc.gov. 2018-03-16. Retrieved 2018-05-04. 10. How harmful is K2/Spice (synthetic marijuana or synthetic cannabinoids)?
- "Statement from FDA warning about significant health risks of contaminated illegal synthetic cannabinoid products that are being encountered by FDA" (Press release). United States Food and Drug Administration. July 19, 2018.
- Fattore, Liana; Fratta, Walter (2011-09-21). "Beyond THC: The New Generation of Cannabinoid Designer Drugs". Frontiers in Behavioral Neuroscience. 5: 60. doi:10.3389/fnbeh.2011.00060. ISSN 1662-5153. PMC 3187647. PMID 22007163.
- Uchiyama, Nahoko; Matsuda, Satoru; Kawamura, Maiko; Kikura-Hanajiri, Ruri; Goda, Yukihiro (2013-07-01). "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology. 31 (2): 223–240. doi:10.1007/s11419-013-0182-9. ISSN 1860-8965.
- Kikura-Hanajiri, Ruri; Uchiyama, Nahoko; Goda, Yukihiro (2011). "Survey of current trends in the abuse of psychotropic substances and plants in Japan". Legal Medicine. 13 (3): 109–115. doi:10.1016/j.legalmed.2011.02.003. ISSN 1344-6223. PMID 21377397.
- Uchiyama, Nahoko; Kawamura, Maiko; Kikura-Hanajiri, Ruri; Goda, Yukihiro (2013). "URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products". Forensic Science International. 227 (1–3): 21–32. doi:10.1016/j.forsciint.2012.08.047. ISSN 0379-0738. PMID 23063179.
- "Synthetic cannabinoids in Europe | www.emcdda.europa.eu". www.emcdda.europa.eu. Retrieved 2018-05-04.
- "New York Bans 'Synthetic Marijuana'". NPR.org. Retrieved 2018-05-04.
- "Synthetic Cannabinoids: The Newest, Almost Illicit Drug of Abuse". www.mdedge.com. Retrieved 2018-05-04.
- "K2 Trend Not Slowing Down". WebMD. Retrieved 2018-05-04.
- Angerer, Verena; Moosman, Bjoern; Franz, Florian; Auwärter, Volker (2015). "5F-cumyl-PINACA in 'e-liquids' for electronic cigarettes – A new type of synthetic cannabinoid in a trendy product" (PDF). Retrieved 14 June 2018.
- O'Brien, Amanda (April 11, 2011). "Miners working high on synthetic grass". The Australian. Retrieved April 17, 2011.
- European Monitoring Centre for Drugs and Drug Addiction (2009). "Understanding the "Spice" Phenomenon" (PDF). EMCDDA 2009 Thematic Paper.
- Pertwee, Roger G (2009-02-02). "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology. 147 (S1): S163–S171. doi:10.1038/sj.bjp.0706406. ISSN 0007-1188. PMC 1760722. PMID 16402100.
- Stefania Crowther; Lois Reynolds; Tilli Tansey, eds. (2010). "The Medicalization of Cannabis" (PDF). Wellcome Witnesses to Twentieth Century Medicine. 40 – via Wellcome Trust Centre for the History of Medicine at UCL.CS1 maint: multiple names: authors list (link) CS1 maint: extra text: authors list (link)
- "Drug Fact Sheet: Marijuana" (PDF). Drug Enforcement Administration.
- "Synthetic Marijuana Deaths Tripled This Year". Newsweek. 2015-06-11. Retrieved 2018-05-05.
- Law, R.; Schier, J.; Martin, C.; Chang, A.; Wolkin, A. (2015). "Notes from the Field: Increase in Reported Adverse Health Effects Related to Synthetic Cannabinoid Use - United States, January-May 2015". Morbidity and Mortality Weekly Report. 64: 618–619.
- "Fake pot that acts real stymies law enforcement". msnbc.com. 2010-02-17. Retrieved 2018-05-05.
- Riederer, A.M.; et al. (2016). "Acute Posionings from Synthetic Cannabinoids - 50 U.S. Toxicology Investigators Consortium Registry Sites, 2010-2015". Morbidity and Mortality Weekly Report. 65 (27): 692–695. doi:10.15585/mmwr.mm6527a2. PMC 4972329. PMID 27413997.
- Fantegrossi, William E.; Moran, Jeffery H.; Radominska-Pandya, Anna; Prather, Paul L. (2014). "Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ9-THC: Mechanism underlying greater toxicity?". Life Sciences. 97 (1): 45–54. doi:10.1016/j.lfs.2013.09.017. ISSN 0024-3205. PMC 3945037. PMID 24084047.
- Brents, Lisa K.; Reichard, Emily E.; Zimmerman, Sarah M.; Moran, Jeffery H.; Fantegrossi, William E.; Prather, Paul L. (2011-07-06). "Phase I Hydroxylated Metabolites of the K2 Synthetic Cannabinoid JWH-018 Retain In Vitro and In Vivo Cannabinoid 1 Receptor Affinity and Activity". PLOS ONE. 6 (7): e21917. doi:10.1371/journal.pone.0021917. ISSN 1932-6203. PMC 3130777. PMID 21755008.
- Behonick, George; Shanks, Kevin G.; Firchau, Dennis J.; Mathur, Gagan; Lynch, Charles F.; Nashelsky, Marcus; Jaskierny, David J.; Meroueh, Chady (2014-10-01). "Four Postmortem Case Reports with Quantitative Detection of the Synthetic Cannabinoid, 5F-PB-22". Journal of Analytical Toxicology. 38 (8): 559–562. doi:10.1093/jat/bku048. ISSN 0146-4760. PMC 4334789. PMID 24876364.
- Shalit, Nadav; Barzilay, Ran; Shoval, Gal; Shlosberg, Dan; Mor, Nofar; Zweigenhaft, Nofar; Weizman, Abraham; Krivoy, Amir (2016-07-05). "Characteristics of Synthetic Cannabinoid and Cannabis Users Admitted to a Psychiatric Hospital". The Journal of Clinical Psychiatry. 77 (8): e989–e995. doi:10.4088/jcp.15m09938. ISSN 0160-6689. PMID 27379411.
- Hurst, Donald; Loeffler, George; McLay, Robert (2011-10-01). "Psychosis Associated With Synthetic Cannabinoid Agonists: A Case Series". American Journal of Psychiatry. 168 (10): 1119. doi:10.1176/appi.ajp.2011.11010176. ISSN 0002-953X. PMID 21969050.
- Pierre, Joseph M. (September 2011). "Cannabis, synthetic cannabinoids, and psychosis risk: What the evidence says" (PDF). Current Psychiatry. 10.
- Every-Palmer, Susanna (2011). "Synthetic cannabinoid JWH-018 and psychosis: An explorative study". Drug and Alcohol Dependence. 117 (2–3): 152–157. doi:10.1016/j.drugalcdep.2011.01.012. ISSN 0376-8716. PMID 21316162.
- Müller, Helge; Sperling, Wolfgang; Köhrmann, Martin; Huttner, Hagen B.; Kornhuber, Johannes; Maler, Juan-Manuel (2010). "The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes". Schizophrenia Research. 118 (1–3): 309–310. doi:10.1016/j.schres.2009.12.001. ISSN 0920-9964. PMID 20056392.
- Freeman MJ, Rose DZ, Myers MA, Gooch CL, Bozeman AC, Burgin WS. Ischemic stroke after use of the synthetic marijuana "spice". Neurology. 2013;81(24):2090-3.
- Wohlfarth, Ariane; Gandhi, Adarsh S.; Pang, Shaokun; Zhu, Mingshe; Scheidweiler, Karl B.; Huestis, Marilyn A. (2014-02-01). "Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry". Analytical and Bioanalytical Chemistry. 406 (6): 1763–1780. doi:10.1007/s00216-014-7668-0. ISSN 1618-2642. PMID 24518903.
- "Synthetic Cannabinoids in Herbal Products" (PDF). United Nations Office on Drugs and Crime.
- "Details for Synthetic cannabinoids". www.unodc.org. Retrieved 2018-05-04.
- "Synthetic Cannabinoids - AACC.org". www.aacc.org. Retrieved 2018-05-04.
- Cannabinoids. Abood, Mary Ellen, 1958-, Pertwee, R. G. (Roger G.). Berlin: Springer. 2005. ISBN 978-3540225652. OCLC 65169431.CS1 maint: others (link)
- Gurney, S.M.R.; Scott, K.S.; Kacinko, S.L.; Presley, B.C.; Logan, B.K. (January 2014). "Pharmacology, Toxicology, and Adverse Effects of Synthetic Cannabinoid Drugs" (PDF). Forensic Science Review. 26: 54–78.
- Banister, Samuel D.; Moir, Michael; Stuart, Jordyn; Kevin, Richard C.; Wood, Katie E.; Longworth, Mitchell; Wilkinson, Shane M.; Beinat, Corinne; Buchanan, Alexandra S. (2015-07-17). "Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA". ACS Chemical Neuroscience. 6 (9): 1546–1559. doi:10.1021/acschemneuro.5b00112. ISSN 1948-7193. PMID 26134475.
- Uchiyama, Nahoko; Matsuda, Satoru; Wakana, Daigo; Kikura-Hanajiri, Ruri; Goda, Yukihiro (2013-01-01). "New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products". Forensic Toxicology. 31 (1): 93–100. doi:10.1007/s11419-012-0171-4. ISSN 1860-8965.
- Hess, Cornelius; Schoeder, Clara T.; Pillaiyar, Thanigaimalai; Madea, Burkhard; Müller, Christa E. (2016-07-01). "Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice". Forensic Toxicology. 34 (2): 329–343. doi:10.1007/s11419-016-0320-2. ISSN 1860-8965. PMC 4929166. PMID 27429655.
- Howlett, A.C.; Champion, T.M.; Wilken, G.H.; Mechoulam, R. (1990). "Stereochemical Effects of 11-OH-delta8-Teatrahydrocannabinol-Dimethylheptyl to Inhibit Adenylate Cyclase and Bind to the Cannabinoid Receptor". Neuropharmacology. 29 (2): 161–165. doi:10.1016/0028-3908(90)90056-W.
- Shohami, Esther; Mechoulam, Raphael (2000-07-01). "Dexanabinol (HU‐211): A nonpsychotropic cannabinoid with neuroprotective properties". Drug Development Research. 50 (3‐4): 211. doi:10.1002/1098-2299(200007/08)50:3/4<211::aid-ddr3>3.0.co;2-g. ISSN 1098-2299.
- Ärzteblatt, Deutscher Ärzteverlag GmbH, Redaktion Deutsches (2009). "Withdrawal Phenomena and Dependence Syndrome After the Consumption of "Spice Gold" (03.07.2009)". Deutsches Arzteblatt International (in German). 106 (27): 464–7. doi:10.3238/arztebl.2009.0464. PMC 2719097. PMID 19652769.
- Auwärter, Volker; Dresen, Sebastian; Weinmann, Wolfgang; Müller, Michael; Pütz, Michael; Ferreirós, Nerea (2009). "'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs?". Journal of Mass Spectrometry. 44 (5): 832–837. doi:10.1002/jms.1558. ISSN 1076-5174. PMID 19189348.
- Milone, Michael C. (2012). "Analytical Techniques used in Therapeutic Drug Monitoring". Therapeutic Drug Monitoring. Elsevier. pp. 49–73. doi:10.1016/b978-0-12-385467-4.00003-8. ISBN 9780123854674.
- Znaleziona, Joanna; Ginterová, Pavlína; Petr, Jan; Ondra, Peter; Válka, Ivo; Ševčík, Juraj; Chrastina, Jan; Maier, Vítězslav (2015). "Determination and identification of synthetic cannabinoids and their metabolites in different matrices by modern analytical techniques – a review". Analytica Chimica Acta. 874: 11–25. doi:10.1016/j.aca.2014.12.055. ISSN 0003-2670. PMID 25910441.
- Spinelli, Eliani; Barnes, Allan J.; Young, Sheena; Castaneto, Marisol S.; Martin, Thomas M.; Klette, Kevin L.; Huestis, Marilyn A. (2014-08-29). "Performance characteristics of an ELISA screening assay for urinary synthetic cannabinoids". Drug Testing and Analysis. 7 (6): 467–474. doi:10.1002/dta.1702. ISSN 1942-7603. PMID 25167963.
- Sobolevsky, Tim; Prasolov, Ilya; Rodchenkov, Grigory (2010). "Detection of JWH-018 metabolites in smoking mixture post-administration urine". Forensic Science International. 200 (1–3): 141–147. doi:10.1016/j.forsciint.2010.04.003. ISSN 0379-0738. PMID 20430547.
- Huppertz, Laura M.; Kneisel, Stefan; Auwärter, Volker; Kempf, Jürgen (2014). "A comprehensive library-based, automated screening procedure for 46 synthetic cannabinoids in serum employing liquid chromatography-quadrupole ion trap mass spectrometry with high-temperature electrospray ionization". Journal of Mass Spectrometry. 49 (2): 117–127. doi:10.1002/jms.3328. ISSN 1076-5174. PMID 24677304.
- Scheidweiler, Karl B.; Jarvis, Michael J. Y.; Huestis, Marilyn A. (2015-01-01). "Nontargeted SWATH acquisition for identifying 47 synthetic cannabinoid metabolites in human urine by liquid chromatography-high-resolution tandem mass spectrometry". Analytical and Bioanalytical Chemistry. 407 (3): 883–897. doi:10.1007/s00216-014-8118-8. ISSN 1618-2642. PMID 25224637.
- 1944-, Baselt, Randall C. (Randall Clint) (2014). Disposition of toxic drugs and chemicals in man (Tenth ed.). Seal Beach, California. ISBN 9780962652394. OCLC 883367655.
- Znaleziona, Joanna; Ginterová, Pavlína; Petr, Jan; Ondra, Peter; Válka, Ivo; Ševčík, Juraj; Chrastina, Jan; Maier, Vítězslav (2015-05-18). "Determination and identification of synthetic cannabinoids and their metabolites in different matrices by modern analytical techniques – a review". Analytica Chimica Acta. 874: 11–25. doi:10.1016/j.aca.2014.12.055. ISSN 0003-2670. PMID 25910441.
- Schlabach, Mark (October 20, 2011). "Sources: LSU players had positive tests". ESPN.com. Retrieved November 4, 2011.
- "ФСКН: от отравления спайсами в российских регионах погибли более 40 человек". TASS. 29 October 2014. Retrieved 13 December 2014.
- Adams, Axel J.; Banister, Samuel D.; Irizarry, Lisandro; Trecki, Jordan; Schwartz, Michael; Gerona, Roy (2017-01-19). ""Zombie" Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York". New England Journal of Medicine. 376 (3): 235–242. doi:10.1056/nejmoa1610300. ISSN 0028-4793. PMID 27973993.
- "Drug 85 Times as Potent as Marijuana Caused a 'Zombielike' State in Brooklyn". Retrieved 2018-09-25.
- "A Drug 85 Times More Potent than THC Caused 'Zombie Outbreak' in New York City | American Council on Science and Health". www.acsh.org. 2017-01-21. Retrieved 2018-09-25.
- "Wisconsinites are smoking rat poison. How did we get here?". Milwaukee Journal Sentinel. Retrieved 2018-09-25.
- "Outbreak Alert: Potential Life-Threatening Vitamin K-Dependent Antagonist Coagulopathy Associated With Synthetic Cannabinoids Use". Centers for Disease Control and Prevention (CDC). Retrieved 2018-05-04.
- "Synthetic Cannabinoids | IDPH". www.dph.illinois.gov. Retrieved 2018-05-04.
- "More Cases of Severe Bleeding in Wisconsin Linked to Synthetic Cannabinoids". Wisconsin Department of Health Services. 2018-09-18. Retrieved 2018-09-25.
- "Bad Batch Of K2 Is Suspected In Dozens Of Overdoses In Connecticut". NPR.org. Retrieved 2018-08-28.
- "New Zealand City Has 10 Synthetic Cannabis Overdoses in 48 Hours • High Times". hightimes.com. 2018-09-21. Retrieved 2018-09-25.
- Whelan, Aubrey. "Two dead, dozens sickened from weekend overdoses in Philly". http://www2.philly.com. Retrieved 2018-09-25. External link in
|work=(help) - "Synthetic marijuana detected in drug sample from Philly overdose spike : Health : WHYY". WHYY. Retrieved 2018-09-25.
- "Kräutermischung "Spice": Gesundheitsministerium stoppt Handel, December 18, 2008". Derstandard.at. December 19, 2008. Retrieved June 19, 2010.
- (AFP) (December 18, 2008). "Austria bans herbal incense 'Spice'". Archived from the original on March 10, 2010. Retrieved June 19, 2010.
- "Gesundheitsministerium setzt Maßnahme zum Verbot von "Spice"". Bmgfj.gv.at. December 18, 2008. Archived from the original on November 21, 2009. Retrieved June 19, 2010.
- "BGBl I Nr. 3 vom January 21, 2009, 22. BtMÄndV vom 19. Jan 2009, S. 49–50" (PDF) (in German). Archived from the original (PDF) on 2009-02-05.
- "Betäubungsmittelrecht: Modedroge Spice wird per Eilverordnung verboten". Spiegel Online. 2009-01-21. Retrieved June 19, 2010.
- "Neue-psychoaktive-Stoffe-Gesetz (NpSG) tritt in Kraft". Bundesgesundheitsministerium. Retrieved 29 October 2017.
- "Arrêté du 24 février 2009 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". Journal Officiel De La République Française. February 27, 2009. Retrieved June 19, 2010.
- "Move on head shops 'not enough' – The Irish Times — Wed, Mar 3, 2010". The Irish Times. March 3, 2010. Retrieved August 24, 2010.
- "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" (in Latvian). likumi.lv. Retrieved June 19, 2010.
- "Saeima steidzami nosaka kriminālatbildību par spaisa paveidu tirgošanas aizlieguma pārkāpumu" (in Latvian). Diena.lv. Retrieved November 12, 2014.
- "Archived copy". Archived from the original on 2009-03-25. Retrieved 2009-04-06.CS1 maint: archived copy as title (link)
- mm, PAP (February 12, 2009). "Sejm za delegalizacją 'dopalaczy'". Wiadomosci.gazeta.pl. Retrieved June 19, 2010.
- "Senat poparł ustawę zakazującą handlu "dopalaczami" – Wiadomości — WP.PL". Wiadomosci.wp.pl. Retrieved June 19, 2010.
- "Oficjalna strona Prezydenta Rzeczypospolitej Polskiej". Archived from the original on July 31, 2009. Retrieved February 20, 2015.
- "OUG 6/2010 pentru modificarea si completarea Legii nr. 143/2000 privind prevenirea si combaterea traficului si consumului ilicit de droguri si pentru completarea Legii nr. 339/2005 privind regimul juridic al plantelor, substantelor si preparatelor stupefiante si psihotrope. Ordonanta de urgenta nr. 6/2010". Dreptonline.ro. August 3, 2000. Retrieved June 19, 2010.
- "О запрещении реализации продукции с содержанием шалфея предсказателей, гавайской розы и голубого лотоса". Rospotrebnadzor.ru. Archived from the original on June 12, 2010. Retrieved June 19, 2010.
- "Постановление от 31 декабря 2009 г. № 1186 О внесении изменений в некоторые постановления Правительства Российской Федерации по вопросам, связанным с оборотом наркотических средств". Government.ru. Archived from the original on September 29, 2010. Retrieved June 19, 2010.
- Кира Латухина (30 October 2014). "Президент предложил запретить "спайсы"". Rossiyskaya Gazeta. Retrieved 13 December 2014.
- "Po ketamíne by sa mala zakázať bylinná zmes Spice". SME.sk. Retrieved June 19, 2010.
- "Wayback Machine". 2 October 2011. Archived from the original on 2 October 2011.
- "Spice, cannabis legal de diseño que se vende como incienso. Noticias de Sociedad". Elconfidencial.com. 2014-11-19. Retrieved 29 October 2017.
- Mac (8 June 2015). "¿El auto cultivo de marihuana se despenaliza en España?". Lamarihuana.com. Retrieved 29 October 2017.
- "Regeringen förbjuder nätdrogen "Spice" from the website of the Government Offices of Sweden". Regeringen.se. Archived from the original on September 10, 2010. Retrieved June 19, 2010.
- Adams, Stephen (February 13, 2009). "Teens in Britain getting legally high on synthetic cannabis banned across Europe". The Daily Telegraph. UK. Retrieved August 13, 2009.
- "Sentetik Cannabinoid (Bonzai)". Uyusturucumaddeler.com. Archived from the original on August 12, 2014. Retrieved 12 August 2014.
- Portal, Gaetan (December 23, 2009). "'Legal high' drugs banned in UK". BBC News. Retrieved May 23, 2010.
- "Psychoactive Substances Act 2016". Legislation.gov.uk. Retrieved 2016-05-25.
- "Controlled Drugs and Substances Act". Laws.justice.gc.ca. November 2, 2017. Retrieved November 7, 2017.
- "Consolidated Index of Drugs and Substances". Isomerdesign.com. April 24, 2010. Retrieved June 19, 2010.
- "Ian Bussières : Le spice: la capitale craque pour les "herbes magiques" | Société". Cyberpresse.ca. Retrieved June 19, 2010.
- "Une pilule une petite granule : Le Spice, un substitut au cannabis?". Pilule.telequebec.tv. Retrieved June 19, 2010.
- "The David Mitchell Rozga Act (S.605 - Dangerous Synthetic Drug Control Act of 2011)". Opencongress.org. Archived from the original on August 23, 2012. Retrieved 2012-09-09.
- Vashi, Sonam (September 26, 2012). "K2 Trend Not Slowing Down".
- "HU-210". USDOJ.gov. Archived from the original on October 7, 2010. Retrieved September 16, 2010.
- Cook, Morgan (February 28, 2011). "Synthetic marijuana illegal as of Tuesday". North County Times. San Diego. Archived from the original on March 3, 2011. Retrieved February 28, 2011.
- "Federal Register - Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I". Federalregister.gov. March 2011. Retrieved February 20, 2015.
- "Drug Scheduling from the US Drug Enforcement Administration website". Justice.gov. Archived from the original on January 12, 2012. Retrieved June 19, 2010.
- Donna Leinwand (May 24, 2010). "Places race to outlaw K2 'Spice' drug". USA Today. Retrieved July 26, 2010.
- Meserve, Jeanne (February 28, 2011). "DEA imposes "emergency" ban to control synthetic marijuana". CNN. Retrieved March 1, 2011.
- "The big business of synthetic highs". TODAY.com. Archived from the original on June 25, 2011. Retrieved February 20, 2015.
- The Associated Press. How major issues fared in Kansas Legislature. CNBC. May 23, 2010. Accessed: May 23, 2010
- Simmons, Andria (May 24, 2010). "Governor signs bill to outlaw synthetic marijuana". ajc.com. Retrieved June 19, 2010.
- "Alabama Coalition Gets Salvia and K2 Banned in Their State". CADCA. May 20, 2010. Retrieved August 24, 2010.
- "TN police, laws try to keep up with synthetic drug chemists". Timesnews.net. Retrieved 29 October 2017.
- Gay, Malcolm. (2010-07-10) Synthetic Marijuana Spurs State Bans. New York Times. Retrieved on August 7, 2011.
- Iowa Code § 124.204(4)(u) (PDF) (defining a Schedule I controlled substance to include "synthetic equivalents of the substances contained in the cannabis plant, or in the resinous extractives of such plant, and synthetic substances, derivatives, and their isomers with similar chemical structure and pharmacological activity to those substances contained in the plant....")
- Gavin Lesnick (July 2, 2010). "Beebe signs emergency ban on K2". Arkansas Online. Arkansas Democrat Gazette. Retrieved July 26, 2010.
- "Synthetic Cannabis — Controlled Substance Information". Retrieved October 16, 2010.
- Daniel Martynowicz. "Illinois Bans Synthetic Cannabinoids". Archived from the original on September 6, 2010. Retrieved September 14, 2010.
- "LARA - Snyder signs bill to classify K2, Spice as Schedule 1 drugs". Michigan.gov. Retrieved February 20, 2015.
- Argus Leader, "New law bans synthetic drugs," by John Hult (February 23rd 2012 - retrieved on May 14th, 2012).
- "Attorney General: Synthetic Drug Enforcement". In.gov. Retrieved February 20, 2015.
- "NC Senate OKs synthetic pot ban". WRAL-TV. 10 February 2011. Retrieved 27 September 2012.
- "Make Synthetic Cannabinoids Illegal" (PDF). General Assembly of North Carolina. 31 January 2011. Retrieved 27 September 2012.
- Allan, David; Fisher, Cindy. Ruling Clarifies 'Legal' Drug Policy. Stars and Stripes via Military.com. May 23, 2010. Accessed: May 23, 2010 Archived June 6, 2010, at the Wayback Machine
- Marines Ban Spice Drug. Military.com. May 23, 2010. Accessed: May 23, 2010 Archived June 6, 2010, at the Wayback Machine
- "Air Force officials ban use and possession of Spice, mood-altering substances. Air Force News. June 17, 2010. Accessed: June 18, 2010". Af.mil. Archived from the original on October 24, 2011. Retrieved June 19, 2010.
- "DEA Moves to Emergency Control Synthetic Marijuana". DEA Public Affairs. U.S. Drug Enforcement Administration. November 24, 2010. Retrieved February 19, 2011.
- Grim, Ryan (November 24, 2010). "K2 Crackdown: DEA Using Emergency Powers To Ban Fake Pot". Huffington Post. Retrieved November 24, 2010.
- the CNN Wire Staff (November 24, 2010). "Feds move to ban 'fake pot'". CNN. Retrieved November 24, 2010.
- Meserve, Jeanne (February 28, 2011). "DEA imposes "emergency" ban to control synthetic marijuana". CNN. Retrieved March 1, 2011.
- "Chile prohibe uso de Spice, La Nacion 24 de Abril 2009". Lanacion.cl. Retrieved June 19, 2010.
- 최연희 (July 2, 2009). 1일부터 '5-메오-밉트’ 등 향정신성의약품 지정 (in Korean). 헬스코리아뉴스. Retrieved February 18, 2010.
- "Dubai Customs foil 126 attempts to smuggle narcotic". Gulfnews.com. Retrieved February 20, 2015.
- Jerga, Josh (June 13, 2011). "Fake cannabis to be outlawed in WA". The Sydney Morning Herald. Retrieved June 15, 2011.
- Bradbury, David (June 18, 2013). "Competition and Consumer Act 2010 - Consumer Protection Notice No. 3 of 2013 - Imposition of Interim Ban on Certain Consumer Goods Containing Synthetic Drug Substances". Retrieved October 28, 2013.
- "Synthetic drug substances - national interim ban". ACCC. Retrieved October 28, 2013.
- "NSW law to ban synthetic drugs passed". September 18, 2013. Retrieved October 28, 2013.
- Coultan, Mark (September 10, 2013). "NSW law to ban synthetic drugs to stop 'legal highs'". The Australian. Retrieved October 28, 2013.
- "Cannabis substitute "Spice" now illegal". TVNZ. April 1, 2009. Retrieved March 2, 2011.
- Heather, Ben (October 6, 2013). "Kronic king gets the green light". Retrieved October 28, 2013.
- Sophie.Ryan@Nzherald.Co.Nz @Sophiealiceryan, Sophie Ryan Business Online Editor (May 7, 2014). "Legal highs banned from midnight". NZ Herald. Retrieved May 8, 2014.CS1 maint: extra text: authors list (link)
- Whitworth, Chris (July 7, 2011). "Kronic ingredients secret to stop industry 'cowboys'". 3 News. Retrieved November 16, 2011.
- 'Herbal High' synthetic Cannabinoid composition, released by ESR July 2011. (PDF) . Retrieved on August 7, 2011.
- "Temporary Class Drug Notices". Ministry of Health NZ. Retrieved February 20, 2015.
- "Conviction for sale of herbal smoking material containing a prescription medicine". Ministry of Health NZ. Retrieved February 20, 2015.
External links
- Erowid
- Synthetic cannabinoid profile at the European Monitoring Centre for Drugs and Drug Addiction
- Interactive model to help understand the structures of synthetic cannabinoids