KM-233
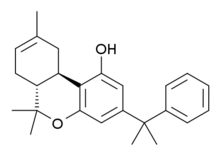
KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor.[1] In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor.[2] Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.[3][4][5][6][7]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H30O2 |
| Molar mass | 362.513 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Krishnamurthy, M; Ferreira, A. M.; Moore Bm, 2nd (2003). "Synthesis and testing of novel phenyl substituted side-chain analogues of classical cannabinoids". Bioorganic & Medicinal Chemistry Letters. 13 (20): 3487–90. doi:10.1016/s0960-894x(03)00729-7. PMID 14505654.
- Duntsch, C; Divi, M. K.; Jones, T; Zhou, Q; Krishnamurthy, M; Boehm, P; Wood, G; Sills, A; Moore Bm, 2nd (2006). "Safety and efficacy of a novel cannabinoid chemotherapeutic, KM-233, for the treatment of high-grade glioma". Journal of Neuro-Oncology. 77 (2): 143–52. doi:10.1007/s11060-005-9031-y. PMID 16314952.
- Nadipuram, A. K.; Krishnamurthy, M; Ferreira, A. M.; Li, W; Moore, B. M. (2003). "Synthesis and testing of novel classical cannabinoids: Exploring the side chain ligand binding pocket of the CB1 and CB2 receptors". Bioorganic & Medicinal Chemistry. 11 (14): 3121–32. doi:10.1016/s0968-0896(03)00238-4. PMID 12818675.
- Durdagi, S; Kapou, A; Kourouli, T; Andreou, T; Nikas, S. P.; Nahmias, V. R.; Papahatjis, D. P.; Papadopoulos, M. G.; Mavromoustakos, T (2007). "The application of 3D-QSAR studies for novel cannabinoid ligands substituted at the C1' position of the alkyl side chain on the structural requirements for binding to cannabinoid receptors CB1 and CB2". Journal of Medicinal Chemistry. 50 (12): 2875–85. doi:10.1021/jm0610705. PMID 17521177.
- Krishnamurthy, M; Gurley, S; Moore Bm, 2nd (2008). "Exploring the substituent effects on a novel series of C1'-dimethyl-aryl Delta8-tetrahydrocannabinol analogs". Bioorganic & Medicinal Chemistry. 16 (13): 6489–500. doi:10.1016/j.bmc.2008.05.034. PMID 18524604.
- Ferreira, A. M.; Krishnamurthy, M; Moore Bm, 2nd; Finkelstein, D; Bashford, D (2009). "Quantitative structure-activity relationship (QSAR) for a series of novel cannabinoid derivatives using descriptors derived from semi-empirical quantum-chemical calculations". Bioorganic & Medicinal Chemistry. 17 (6): 2598–606. doi:10.1016/j.bmc.2008.11.059. PMID 19250829.
- Brogi, S; Corelli, F; Di Marzo, V; Ligresti, A; Mugnaini, C; Pasquini, S; Tafi, A (2011). "Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2". European Journal of Medicinal Chemistry. 46 (2): 547–55. doi:10.1016/j.ejmech.2010.11.034. PMID 21183257.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.