AH-7921
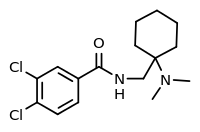
AH-7921 is an opioid analgesic drug selective for the μ-opioid receptor, having around 90% the potency of morphine when administered orally.[1][2][3] It was discovered in the 1970s[4] by a team at Allen and Hanburys located in the United Kingdom.[5] The drug is considered a new psychoactive substance (NPS) in which it is synthetically created in laboratories to mimic that of controlled substances. The substance has also been sold on the internet since 2012 as a "research chemical".[6] When sold online it may be called the alternative name doxylam, not to be confused with doxylamine.[7] AH-7921 has never progressed to clinical trials.[8] The DEA is not aware of any medical usage in the United States, and has not insisted the Health and Human Services department (HHS) to conduct any medical research of the substances uses.[5]
 | |
| Clinical data | |
|---|---|
| Other names | AH-7921 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H22Cl2N2O |
| Molar mass | 329.265 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Types of administration
- Intravenous injection
- Nasal insufflation
- Oral or rectal (when in the form of a powder, tablet, or capsule)[6]
- Sublingual application
Side effects and withdrawal
With doses that usually range from 10 to 150 mg, users are likely to experience effects similar to heroin, morphine, and fentanyl such as euphoria and respiratory depression.[6][8] When an overdose occurs users often experience tachycardia, hypertension, and seizures.[7] Mice, dogs, and monkeys, have been used in tests which showed the drug was almost equivalently potent to morphine, and had a very steep dose response curve.[9] Rats given 20 mg doses three times a day for five days, experienced withdrawal symptoms similar to other opioids. Reports have shown users to experience depression and insomnia when withdrawing from this drug.[6]
Chemistry
AH-7921 is commonly found as an off-white solid with a melting point between 215–216 degrees Celsius.[6] It is one single covalently bonded unit with 4 rotatable bonds. It also has two hydrogen bond acceptors, and one hydrogen bond donor.[10]
Use
Although AH-7921 was extensively studied in vitro and in animals, though not in humans, by the developing company, it was never sold commercially for medical use. In 2013, AH-7921 was discovered to have been used as an active ingredient in "synthetic cannabis" products in Japan.[11] In October 2015, two horses (Bossmon and Literata) trained by owner-trainer Roy Sedlacek tested positive for AH-7921 at Belmont Park racetrack.[12] Norway and Iceland use this substance as an analytical reference standard in which it can be ordered online from chemical suppliers; however, labels are required to state that it is not for human consumption thus conforming to the law.[6] Within ten months from 2013 - 2014, there have been serious fatalities reported from this drug from four different countries.[9] The first fatality was a 19-year-old male who had a 3.9 mg/L AH-7921 concentration of heart blood leading medical examiners to confirm the death was an opioid intoxication.[13] In another case, a 22-year-old woman was found dead with a femoral blood to AH-7921 concentration of 450μg/L.[14]
A 2018 review of published case reports found a total of 14 cases, of which 13 resulted in death.[15] The oral route of administration was reported in two cases, and most cases reported use of concomitant pharmaceutical agents. Postmortem autopsies found that pulmonary edema was the most common finding, with nine of the cases having heavier lungs. Overall, fatalities occurred with low and high concentrations of AH-7921 in femoral blood.[15]
Legality
AH-7921 was made a Prohibited Substance (Schedule 9 of the Standard for the Uniform Scheduling of Medicines and Poisons) in Australia in May 2014.[16] Although this amendment was repealed in June 2014,[17] which simply means the amendment document ceases, but the actual scheduling is permanent as part of the main document (all SUSMP amendments cease after a few weeks). It may, however, still be a banned import.
AH-7921 has been illegal to distribute in Israel since December 2013.[18]
In the UK, AH-7921 was included as a Class A drug in January 2015 as part of The Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014.[19]
In Brazil, AH-7921 has been an illegal drug since May 2015.[20]
As of October 2015 AH-7921 is a controlled substance in China.[21]
AH-7921 is banned in the Czech Republic.[22]
In the United States, AH-7921 was placed into Schedule I of the Controlled Substances Act on May 16, 2016 due to its lack of medical use.[5][23] Furthermore, any person who wishes to manufacture, distribute, import, export, research, and educate using the substance, must be registered by the Drug Enforcement Administration.[5]
The Canadian Controlled Drugs and Substances Act was amended in 2016 to include the substance as a Schedule I substance. Possession without legal authority can result in maximum 7 years imprisonment. Further, Health Canada amended the Food and Drug Regulations in May, 2016 to classify AH-7921 as a restricted drug.[24] Only those with a law enforcement agency, person with an exemption permit or institutions with Minister's authorization may possess the drug in Canada.
See also
References
- Brittain, R. T.; Kellett, D. N.; Neat, M. L.; Stables, R. (September 1973). "Proceedings: Anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides". British Journal of Pharmacology. 49 (1): 158P–159P. doi:10.1111/j.1476-5381.1973.tb08279.x. PMC 1776456. PMID 4207044.
- Hayes, A. G.; Tyers, M. B. (July 1983). "Determination of receptors that mediate opiate side effects in the mouse". British Journal of Pharmacology. 79 (3): 731–736. doi:10.1111/j.1476-5381.1983.tb10011.x. PMC 2044905. PMID 6317119.
- N. J. Harper; G. B. A. Veitch; D. G. Wibberley (November 1974). "1-(3,4-Dichlorobenzamidomethyl)cyclohexyldimethylamine and related compounds as potential analgesics". Journal of Medicinal Chemistry. 17 (11): 1188–1193. doi:10.1021/jm00257a012. PMID 4416926.
- US patent 3975443, Harper, N.; Veitch, G., "1-(3,4-DICHLOROBENZAMIDOMETHYL)-CYCLOHEXYLDIMETHYLAMINE", issued 1976-08-17, assigned to Allen & Hanburys
- "2016 - Final Order: Placement of AH-7921 Into Schedule I". www.deadiversion.usdoj.gov. Retrieved 2017-12-03.
- Katselou, Maria; Papoutsis, Ioannis; Nikolaou, Panagiota; Spiliopoulou, Chara; Athanaselis, Sotiris (2015). "AH-7921: the list of new psychoactive opioids is expanded". Forensic Toxicology. 33 (2): 195–201. doi:10.1007/s11419-015-0271-z. ISSN 1860-8965. PMC 4525185. PMID 26257832.
- Zawilska, Jolanta B. (2017-06-30). "An Expanding World of Novel Psychoactive Substances: Opioids". Frontiers in Psychiatry. 8: 110. doi:10.3389/fpsyt.2017.00110. ISSN 1664-0640. PMC 5492455. PMID 28713291.
- "The New Wave of Designer Drugs | Forensic Scholars Today". Concordia University, St. Paul Online. 2017-01-30. Retrieved 2017-12-03.
- Wohlfarth, Ariane; Scheidweiler, Karl B.; Pang, Shaokun; Zhu, Mingshe; Castaneto, Marisol; Kronstrand, Robert; Huestis, Marilyn A. (August 2016). "Metabolic characterization of AH-7921, a synthetic opioid designer drug: in vitro metabolic stability assessment and metabolite identification, evaluation of in silico prediction, and in vivo confirmation". Drug Testing and Analysis. 8 (8): 779–791. doi:10.1002/dta.1856. ISSN 1942-7603. PMC 4562414. PMID 26331297.
- Pubchem. "AH-7921". pubchem.ncbi.nlm.nih.gov. Retrieved 2017-12-06.
- Nahoko Uchiyama; Satoru Matsuda; Maiko Kawamura; Ruri Kikura-Hanajiri; Yukihiro Goda (July 2013). "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology. 31 (2): 223–240. doi:10.1007/s11419-013-0182-9.
- "Two horses test positive for designer drug". Racing Post. Retrieved 29 April 2016.
- Vorce, Shawn P.; Knittel, Jessica L.; Holler, Justin M.; Magluilo, Joseph; Levine, Barry; Berran, Philip; Bosy, Thomas Z. (May 2014). "A fatality involving AH-7921". Journal of Analytical Toxicology. 38 (4): 226–230. doi:10.1093/jat/bku011. ISSN 1945-2403. PMID 24523294.
- "Two fatalities associated with synthetic opioids: AH-7921 and MT-45 - PubAg". pubag.nal.usda.gov. Retrieved 2017-12-03.
- Rambaran, Kerry Anne; Amin, Zubair M.; Fleming, Steven W.; Chacko, Liza; Alzghari, Saeed K. (July 2018). "AH-7921: A review of previously published reports". Proceedings (Baylor University. Medical Center). 31 (3): 303–306. doi:10.1080/08998280.2018.1465320. ISSN 0899-8280. PMC 5997081. PMID 29904293.
- Final decisions and reasons for decisions by delegates of the Secretary to the Department of Health
- Poisons Standard Amendment No. 2 of 2014
- פקודת הסמים המסוכנים נוסח חדש, תשל"ג-1973
- The Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014
- Resolução da Diretoria Colegiada - RDC nº 87 de 28/06/2016
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.
- "Schedules of Controlled Substances: Placement of AH-7921 Into Schedule I". Drug Enforcement Administration. 14 April 2016.
- Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18)