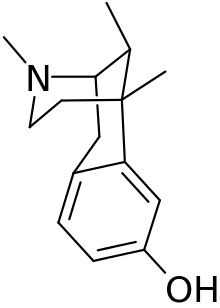
Metazocine
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects,[1] mediated through a mixed agonist–antagonist action[2] at the mu opioid receptor,[3] its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors (where it is a high-efficacy agonist)[4] and/or sigma receptors.[5][6]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.998 |
| Chemical and physical data | |
| Formula | C15H21NO |
| Molar mass | 231.333 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Metazocine is in Schedule II of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9240 with a 19 gram aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.81 for the hydrochloride and 0.74 for the hydrobromide.[7] It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as is morphine.
Syntheses
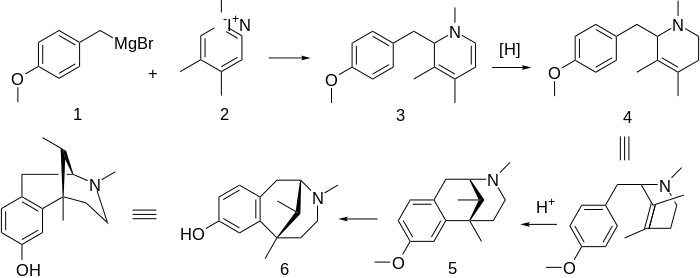
The prototype benzomorphan, metazocine (6), can be obtained from a variation of the morphinan synthesis.
Thus, reaction of the Grignard reagent from p-methoxybenzyl chloride (1) with the lutidine methiodide (2) affords the benzylated dihydropyridine (3). Reduction of the enamine π-bond leads to the tetrahydropyridine (4). Cyclization by means of acid leads directly to the benzomorphan ring system (5). Demethylation of the aromatic ring system affords the phenol. Although this last compound is in fact a relatively potent analgesic, it is not available commercially as a drug.
See also
- Phenazocine
- Pentazocine
- Cyclazocine
- Org 6582, a functional MAT inhibitor that is otherwise analogous in structure to the parent compound of article
References
- Hori M, Ban M, Imai E, Iwata N, Suzuki Y, Baba Y, Morita T, Fujimura H, Nozaki M, Niwa M. Novel nonnarcotic analgesics with an improved therapeutic ratio. Structure-activity relationships of 8-(methylthio)- and 8-(acylthio)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocines. Journal of Medicinal Chemistry. 1985 Nov;28(11):1656-61.
- Berzetei-Gurske I, Loew GH. The novel antagonist profile of (-)metazocine. Progress in Clinical and Biological Research. 1990;328:33-6.
- Gharagozlou P, Demirci H, David Clark J, Lameh J. Activity of opioid ligands in cells expressing cloned mu opioid receptors. BMC Pharmacology. 2003 Jan 4;3:1.
- Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacology. 2006 Jan 25;6:3.
- Shannon HE. Pharmacological analysis of the phencyclidine-like discriminative stimulus properties of narcotic derivatives in rats. Journal of Pharmacology and Experimental Therapeutics. 1982 Jul;222(1):146-51.
- Slifer BL, Balster RL, May EL. Reinforcing and phencyclidine-like stimulus properties of enantiomers of metazocine. Pharmacology Biochemistry and Behavior. 1986 Oct;25(4):785-9.
- http://www.deadiversion.usdoj.gov/fed_regs/quotas/2014/fr0825.htm
- May, E. L.; Fry, E. M. (1957). "Structures Related to Morphine. VIII. Further Syntheses in the Benzomorphan Series*1,2". The Journal of Organic Chemistry. 22 (11): 1366. doi:10.1021/jo01362a017.
- May, E. L.; Ager, J. H. (1959). "Structures Related to Morphine. XI.1Analogs and a Diastereoisomer of 2'-Hydroxy-2,5,9-trimethyl-6,7-benzomorphan". The Journal of Organic Chemistry. 24 (10): 1432. doi:10.1021/jo01092a011.