25B-NBOH
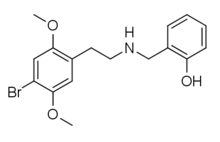
25B-NBOH (2C-B-NBOH, NBOH-2C-B) is a derivative of the phenethylamine derived hallucinogen 2C-B which has been sold as a designer drug. It acts as a potent serotonin receptor agonist with similar affinity to the better-known compound 25B-NBOMe at 5-HT2A and 5-HT2C receptors with pKis values of 8.3 and 9.4, respectively.[1][2][3][4][5][6]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C17H20BrNO3 |
| Molar mass | 365.06 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Legal status
Sweden
The Riksdag added 25B-NBOH to Narcotic Drugs Punishments Act under swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of January 26, 2016, published by Medical Products Agency (MPA) in regulation HSLF-FS 2015:35 listed as 25B-NBOH, and 2-([2-(4-bromo-2,5-dimetoxifenyl)etylamino]metyl)fenol.[7]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[8]
Analogues and derivatives
Analogues and derivatives of 2C-B:
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
Other:
- 2C-B-AN
- 2C-B-BUTTERFLY
- 2C-B-DragonFLY
- 2CB-5-hemifly
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- TCB-2 (2C-BCB)
References
- Heim, Ralf (19 March 2004). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur". Freie Universität Berlin. Retrieved 27 June 2015.
- Hansen, M.; Phonekeo, K.; Paine, J. S.; Leth-Petersen, S.; Begtrup, M.; Bräuner-Osborne, H.; Kristensen, J. L. (2014). "Synthesis and Structure-Activity Relationships of N-Benzyl Phenethylamines as 5-HT2A/2C Agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Martin Hansen (2011). "Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain" (PDF). University of Copenhagen. Archived from the original (PDF) on 4 July 2015. Retrieved 27 June 2015.
- Ettrup, A.; Hansen, M.; Santini, M. A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M. M.; Madsen, J. (2010). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–693. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- Silva, M. E.; Heim, R.; Strasser, A.; Elz, S.; Dove, S. (2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-aided Molecular Design. 25 (1): 51–66. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982.
- Braden, M. R.; Parrish, J. C.; Naylor, J. C.; Nichols, D. E. (2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe3396.51 and Phe3406.52 with superpotent N-benzyl phenethylamine agonists". Molecular Pharmacology. 70 (6): 1956–1964. doi:10.1124/mol.106.028720. PMID 17000863.
- https://lakemedelsverket.se/upload/lvfs/HSLF-FS/HSLFS-FS_2015_35.pdf
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|