Dexanabinol
Dexanabinol (HU-211 or ETS2101[1]) is a synthetic cannabinoid derivative in development by e-Therapeutics plc. It is the "unnatural" enantiomer of the potent cannabinoid agonist HU-210.[2] Unlike other cannabinoid derivatives, HU-211 does not act as a cannabinoid receptor agonist, but instead has NMDA antagonist effects.[3] It therefore does not produce cannabis-like effects, but is anticonvulsant and neuroprotective, and is widely used in scientific research as well as currently being studied for applications such as treating head injury, stroke, or cancer.[4][5][6] It was shown to be safe in clinical trials[7] and is currently undergoing Phase I trials for the treatment of brain cancer[8] and advanced solid tumors.[9]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
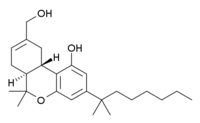
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.201.022 |
| Chemical and physical data | |
| Formula | C25H38O3 |
| Molar mass | 386.567 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Clinical Trials
Dexanabinol has been studied in IV administration and oral dosing.[10] e-Therapeutics is evaluating the compound in clinical trials for brain and solid cancers.[11] Phase II studies are planned based on the results of the current trials.
A phase 1b study for hepatocellular carcinoma and pancreatic cancer was started in 2015.[12]
Legal status
HU-211 is not listed in the schedules set out by the United Nations' Single Convention on Narcotic Drugs from 1961 nor their Convention on Psychotropic Substances from 1971,[13] so the signatory countries to these international drug control treaties are not required by said treaties to control HU-211.
United States
HU-211 is not listed in the list of scheduled controlled substances in the USA.[14] It is therefore not scheduled at the federal level in the United States, but it is possible that HU-211 could legally be considered an analog of Delta-8-THC (one of the THC isomers which is in Schedule I under the designation of "Tetrahydrocannabinols"), and therefore sales or possession could potentially be prosecuted under the Federal Analogue Act.[15]
Alabama
HU-211 is a Schedule I controlled substance in Alabama.[16]
10.
(6aS,10aS)-9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol, some trade or other names: HU-211, Dexanabinol.
Florida
HU-211 is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[17] It is listed twice under the hallucinogic substances subcategory:
| “ | (c) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following hallucinogenic substances or that contains any of their salts, isomers, including optical, positional, or geometric isomers, homologues, nitrogen-heterocyclic analogs, esters, ethers, and salts of isomers, homologues, nitrogen-heterocyclic analogs, esters, or ethers, if the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation or class description:
... 130. HU-211 ((6aS,10aS)-9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol). ... 190. Synthetic Cannabinoids.—Unless specifically excepted or unless listed in another schedule or contained within a pharmaceutical product approved by the United States Food and Drug Administration, any material, compound, mixture, or preparation that contains any quantity of a synthetic cannabinoid found to be in any of the following chemical class descriptions, or homologues, nitrogen-heterocyclic analogs, isomers (including optical, positional, or geometric), esters, ethers, salts, and salts of homologues, nitrogen-heterocyclic analogs, isomers, esters, or ethers, whenever the existence of such homologues, nitrogen-heterocyclic analogs, isomers, esters, ethers, salts, and salts of isomers, esters, or ethers is possible within the specific chemical class or designation. Since nomenclature of these synthetically produced cannabinoids is not internationally standardized and may continually evolve, these structures or the compounds of these structures shall be included under this subparagraph, regardless of their specific numerical designation of atomic positions covered, if it can be determined through a recognized method of scientific testing or analysis that the substance contains properties that fit within one or more of the following categories: a. Tetrahydrocannabinols.—Any tetrahydrocannabinols naturally contained in a plant of the genus Cannabis, the synthetic equivalents of the substances contained in the plant or in the resinous extracts of the genus Cannabis, or synthetic substances, derivatives, and their isomers with similar chemical structure and pharmacological activity, including, but not limited to, Delta 9 tetrahydrocannabinols and their optical isomers, Delta 8 tetrahydrocannabinols and their optical isomers, Delta 6a,10a tetrahydrocannabinols and their optical isomers, or any compound containing a tetrahydrobenzo[c]chromene structure with substitution at either or both the 3-position or 9-position, with or without substitution at the 1-position with hydroxyl or alkoxy groups, including, but not limited to: ... (III) HU-211 ((6aS,10aS)-9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol). |
” |
Vermont
Effective January 1, 2016, HU-211 is a regulated drug in Vermont designated as a "Hallucinogenic Drug."[18]
“Hallucinogenic Drug” means those specified in Section 7 of this rule including stramonium, mescaline or peyote, lysergic acid diethylamide, and psilocybin, and all synthetic equivalents of chemicals contained in resinous extractives of Cannabis sativa, or any salts or derivatives or compounds of any preparations or mixtures thereof, and any other substance having a hallucinogenic effect in the regulations adopted by the Board of Health under 18 V.S.A.§ 4202.
...
• HU-211, Dexanabinol; (6aS, 10aS)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2- yl)- 6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol; OR (6aS, 10aS)-9-(hydroxymethyl)- 6,6-dimethyl- 3-(2-methyloctan-2-yl)-6a,7,10,10a- tetrahydrobenzo[c]chromen-1-ol
References
- "e-therapeutics Clinical Development Pipeline". Archived from the original on 2013-01-26. Retrieved 2012-10-23.
- Pop E (September 2000). "Nonpsychotropic synthetic cannabinoids". Current Pharmaceutical Design. 6 (13): 1347–60. doi:10.2174/1381612003399446. PMID 10903397.
- Feigenbaum JJ; et al. (December 1989). "Nonpsychotropic cannabinoid acts as a functional N-methyl-D-aspartate receptor blocker". Proceedings of the National Academy of Sciences of the United States of America. 86 (23): 9584–7. doi:10.1073/pnas.86.23.9584. PMC 298542. PMID 2556719.
- Biegon A; Joseph AB (August 1995). "Development of HU-211 as a neuroprotectant for ischemic brain damage". Neurological Research. 17 (4): 275–80. PMID 7477742.
- Darlington CL (October 2003). "Dexanabinol: a novel cannabinoid with neuroprotective properties". IDrugs : the Investigational Drugs Journal. 6 (10): 976–9. PMID 14534855.
- Vink R; Nimmo AJ (January 2009). "Multifunctional drugs for head injury". Neurotherapeutics. 6 (1): 28–42. doi:10.1016/j.nurt.2008.10.036. PMC 5084254. PMID 19110197.
- Maas AI; et al. (January 2006). "Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial". Lancet Neurol. 5 (1): 38–45. doi:10.1016/S1474-4422(05)70253-2. PMID 16361021.
- University of California, San Diego "Synthetic Cannabinoid May Be Used as Brain Cancer Treatment". (28 September 2012) Laboratory Equipment. Retrieved 28 September 2012.
- "A Phase 1 Study of Dexanabinol in Patients With Advanced Solid Tumours". ClinicalTrials.gov. NIH. January 26, 2015.
- "e-Therapeutics Reports Progress in ETS2101 Phase 1a and Oral Dosing Studies" (PDF). 18 December 2014. Archived from the original (PDF) on 5 February 2015.
- "Clinical Development Pipeline". Archived from the original on February 5, 2015. Retrieved Feb 5, 2015.
- "A Study of Dexanabinol in Combination With Chemotherapy in Patients With Advanced Tumours - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2015-09-18.
- UN International Drug Control Conventions
- §1308.11 Schedule I.
- Erowid Analog Law Vault : Federal Controlled Substance Analogue Act Summary
- "Alabama Senate Bill 333 - Controlled substances, Schedule I, additional synthetic controlled substances and analogue substances included in, trafficking in controlled substance analogues, requisite weight increased, Secs. 13A-12-231, 20-2-23 am'd". March 2014. Retrieved 2 February 2017.
- Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL
- Vermont DOH - Regulated Drug Rule 2016 .PDF