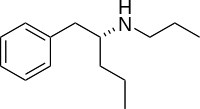
Phenylpropylaminopentane
(-)-1-Phenyl-2-propylaminopentane (also known as (-)-PPAP and N,α-dipropylphenethylamine)[1][2][3] is a stimulant of the substituted phenethylamine class and a derivative of Selegiline.[4] When compared with Selegiline and other substituted phenethylamines (-)-PPAP has a notably different mechanism of action and pharmacological effect.[5]
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C14H23N |
| Molar mass | 205.345 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
(-)-PPAP is classified as a monoaminergic activity enhancer that stimulates the impulse propagation mediated transmitter release of the neurotransmitters dopamine, norepinephrine and serotonin in the brain. Unlike stimulants such as amphetamine, which release a flood of monoamine neurotransmitters in an uncontrolled manner, (-)-PPAP instead only increases the amount of neurotransmitters that get released when a neuron is stimulated by receiving an impulse from a neighbouring neuron. Both amphetamine and (-)-PPAP promote the release of monoamines and deuteramines, however while amphetamine causes neurons to dump neurotransmitter stores into the synapse regardless of external input, (-)-PPAP does not influence the pattern of neurotransmitter release and instead releases a larger amount of neurotransmitters than normal.[6]
(-)-PPAP has no monoamine oxidase inhibitory activity.[7]
See also
References
- Jozsef Knoll (15 June 1993). "Patent US 5220068 - Psychostimulant agent".
- Fumio Yoneda (10 April 2001). "Patent US 6214859 - Ethylamine derivatives".
- Jozsef Knoll (24 December 2001). "Patent US 5075338 - Method of treatment of learning deficiency".
- Knoll J, Knoll B, Török Z, Timár J, Yasar S (March–April 1992). "The pharmacology of 1-phenyl-2-propylamino-pentane (PPAP), a deprenyl-derived new spectrum psychostimulant". Archives Internationales de Pharmacodynamie et de Thérapie. 316 (316): 5–29. PMID 1356324.
- Knoll J, Knoll B, Török Z, Timár J, Yasar S (March–April 1992). "The pharmacology of 1-phenyl-2-propylamino-pentane (PPAP), a deprenyl-derived new spectrum psychostimulant". Archives Internationales de Pharmacodynamie et de Thérapie. 316 (316): 5–29. PMID 1356324.
- Joseph Knoll; Ildikó Miklya; Berta Knoll; Raissa Markó; Károly Kelemen (February 1996). "(−)Deprenyl and (−)1-phenyl-2-propylaminopentane, [(−) PPAP] act primarily as potent stimulants of action potential — transmitter release coupling in the catecholaminergic neurons". Life Sciences. 58 (10): 817–827. doi:10.1016/0024-3205(96)00014-8. PMID 8602114.
- G. Csaba; P. Kovács; Éva Pállinger (January–February 2006). "Acute and delayed effect of (−) deprenyl and (−) 1-phenyl-2-propylaminopentane (PPAP) on the serotonin content of peritoneal cells (white blood cells and mast cells)". Cell Biochemistry and Function. 24 (1): 49–53. doi:10.1002/cbf.1183. PMID 15584092.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||