2C (psychedelics)
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring.[1] Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds.[2] Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL (Phenethylamines i Have Known And Loved). Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.[3]
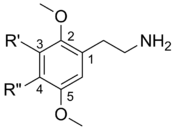
| Nomenclature | R3 | R4 | 2D Structure | 3D Structure |
|---|---|---|---|---|
| 2C-B | H | Br | 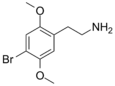 |
 |
| 2C-Bn | H | CH2C6H5 | 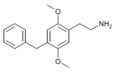 |
|
| 2C-Bu | H | CH2CH2CH2CH3 | 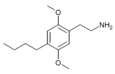 |
|
| 2C-C | H | Cl | 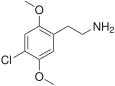 |
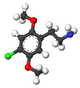 |
| 2C-C-3 [4] | Cl | Cl | 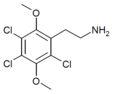 |
|
| 2C-CN | H | C≡N | 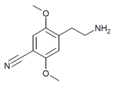 |
|
| 2C-CP | H | C3H5 | 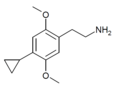 |
|
| 2C-D | H | CH3 | 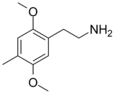 |
 |
| 2C-E | H | CH2CH3 | 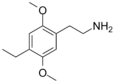 |
 |
| 2C-EF | H | CH2CH2F | 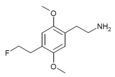 |
|
| 2C-F | H | F | 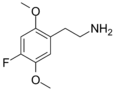 |
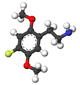 |
| 2C-G | CH3 | CH3 | 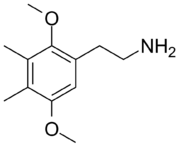 |
 |
| 2C-G-1 | CH2 | 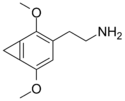 |
||
| 2C-G-2 | (CH2)2 | 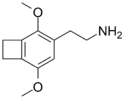 |
||
| 2C-G-3 | (CH2)3 | 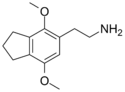 |
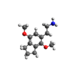 | |
| 2C-G-4 | (CH2)4 | 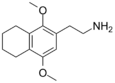 |
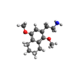 | |
| 2C-G-5 | (CH2)5 | 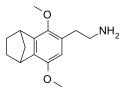 |
||
| 2C-G-6 | (CH2)6 | 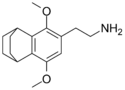 |
||
| 2C-G-N | (CH)4 | 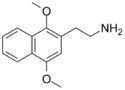 |
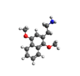 | |
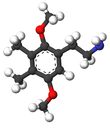
| 2C-H | H | H | 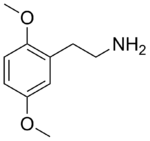 |
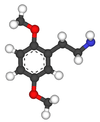 |
| 2C-I | H | I | 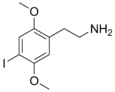 |
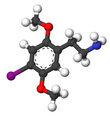 |
| 2C-iP | H | CH(CH3)2 | 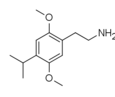 |
|
| 2C-N | H | NO2 | 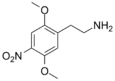 |
 |
| 2C-NH2 | H | NH2 | 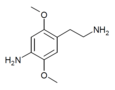 |
|
| 2C-PYR | H | Pyrrolidine | 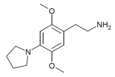 |
|
| 2C-PIP | H | Piperidine | 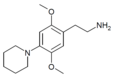 |
|
| 2C-O | H | OCH3 | 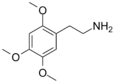 |
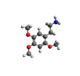 |
| 2C-O-4 | H | OCH(CH3)2 | 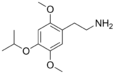 |
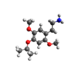 |
| 2C-MOM [5] | H | CH2OCH3 | 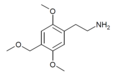 |
|
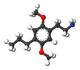
| 2C-P | H | CH2CH2CH3 | 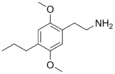 |
 |
| 2C-Ph | H | C6H5 | 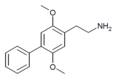 |
|
| 2C-Se | H | Se CH3 | 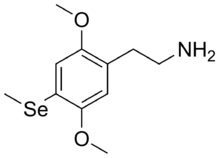 |
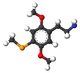 |
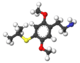
| 2C-T | H | SCH3 | 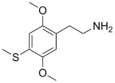 |
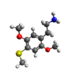 |
| 2C-T-2 | H | SCH2CH3 | 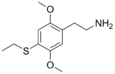 |
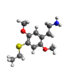 |
| 2C-T-3[6] | 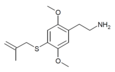 |
|||
| 2C-T-4 | H | SCH(CH3)2 | 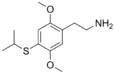 |
 |
| 2C-T-5[6] | 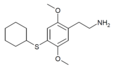 |
|||
| 2C-T-6[6] | 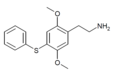 |
|||
| 2C-T-7 | H | S(CH2)2CH3 | 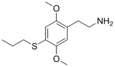 |
 |
| 2C-T-8 | H | SCH2CH(CH2)2 | 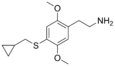 |
 |
| 2C-T-9[6] | 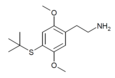 |
|||
| 2C-T-10[6] | 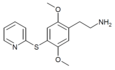 |
|||
| 2C-T-11[6] | 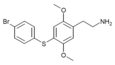 |
|||
| 2C-T-12[6] | 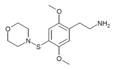 |
|||
| 2C-T-13 | H | S(CH2)2OCH3 | 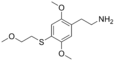 |
 |
| 2C-T-14[7] | 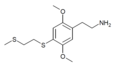 |
|||
| 2C-T-15 | H | SCH(CH2)2 | 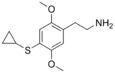 |
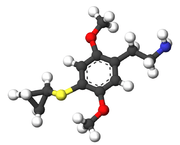 |
| 2C-T-16 [8] | H | SCH2CH=CH2 | 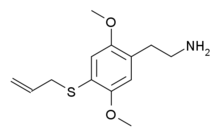 |
|
| 2C-T-17 | H | SCH(CH3)CH2CH3 | 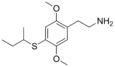 |
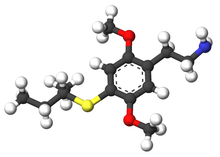 |
| 2C-T-18[7] | 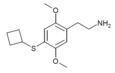 |
|||
| 2C-T-19 | H | SCH2CH2CH2CH3 | 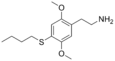 |
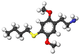 |
| 2C-T-21 | H | S(CH2)2F | 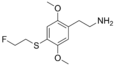 |
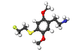 |
| 2C-T-21.5[7] | 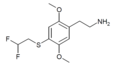 |
|||
| 2C-T-22[7] | 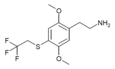 |
|||
| 2C-T-23[7] | 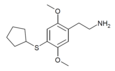 |
|||
| 2C-T-24[7] | 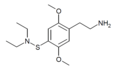 |
|||
| 2C-T-25[7] | 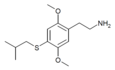 |
|||
| 2C-T-27[7] | 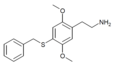 |
|||
| 2C-T-28[7] | 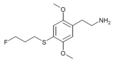 |
|||
| 2C-T-30[7] | 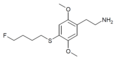 |
|||
| 2C-T-31[7] | 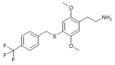 |
|||
| 2C-T-32[7] | 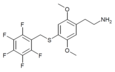 |
|||
| 2C-T-33[7] | 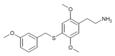 |
|||
| 2C-DFM [9]:770 | H | CHF2 | 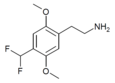 |
|
| 2C-TFM | H | CF3 | 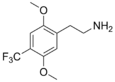 |
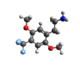 |
| 2C-TFE | H | CH2CF3 | 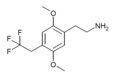 |
|
| 2C-YN | H | C≡CH | 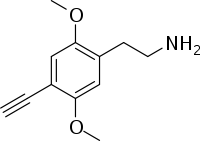 |
|
| 2C-V | H | CH=CH2 | 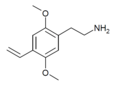 |
|
| 2C-AL | H | CH2CH=CH2 | 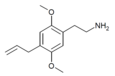 |
|
Legality
Canada
As of October 12, 2016, the 2C-x family of substituted phenethylamines is a controlled substances (Schedule III) in Canada.[10]
See also
References
- Alexander Shulgin, Tania Manning and Paul F Daley. The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press, 2011. ISBN 978-0-9630096-3-0
- Daniel Trachsel, David Lehmann and Christoph Enzensperger. Phenethylamine Von der Struktur zur Funktion, pp 762-810. Nachtschatten Verlag AG, 2013. ISBN 978-3-03788-700-4
- Shulgin, Alexander; Ann Shulgin (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- Takahashi M, Nagashima M, Suzuki J, Seto T, Yasuda I, Yoshida T. Creation and application of psychoactive designer drugs data library using liquid chromatography with photodiode array spectrophotometry detector and gas chromatography–mass spectrometry. Talanta, 15 Feb 2009, 77(4): 1245–1272. doi:10.1016/j.talanta.2008.07.062
- Leth-Petersen S, Petersen IN, Jensen AA, Bundgaard C, Bæk M, Kehler J, Kristensen JL. 5-HT2A/5-HT2C receptor pharmacology and intrinsic clearance of N-benzylphenethylamines modified at the primary site of metabolism. ACS Chem. Neurosci., 16 Nov 2016, 7 (11), 1614–1619. doi:10.1021/acschemneuro.6b00265
- "Shulgin's Sulfur Symphony – Part I". countyourculture. 15 January 2011.
- "Shulgin's Sulfur Symphony – Part I". countyourculture. 15 January 2011.
- Daniel Trachsel (2003). "Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines)". Helvetica Chimica Acta. 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
- Daniel Trachsel; David Lehmann & Christoph Enzensperger (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.
- "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Canada Gazette. April 15, 2016. Retrieved August 28, 2016.
|
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| TAAR1 |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAAR2 |
| ||||||||||
| TAAR5 |
| ||||||||||
† References for all endogenous human TAAR1 ligands are provided at List of trace amines
‡ References for synthetic TAAR1 agonists can be found at TAAR1 or in the associated compound articles. For TAAR2 and TAAR5 agonists and inverse agonists, see TAAR for references.
| |||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|