Substituted phenylmorpholine
Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of phenylmorpholine or of the psychostimulant drug phenmetrazine.
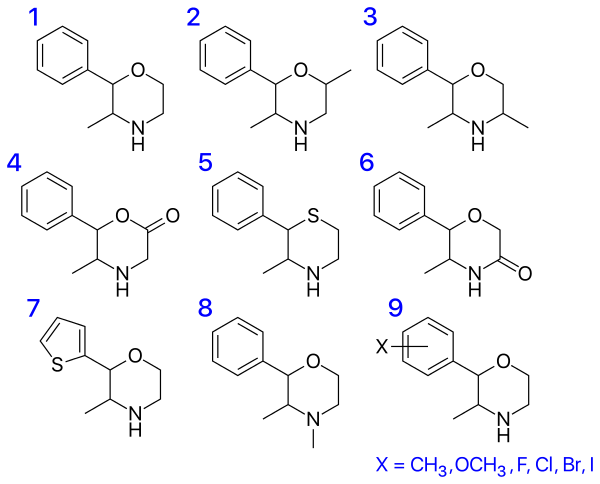
Various phenmetrazine derivatives
-3%2C4-dimethyl-2-phenylmorpholine.png)
The 2S,3S isomer of phendimetrazine (i.e. (2S,3S)-3,4-dimethyl-2-phenylmorpholine)
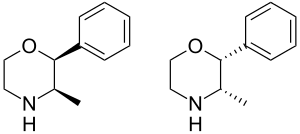
The (+)-enantiomer & (−)-enantiomer of pseudophenmetrazine.
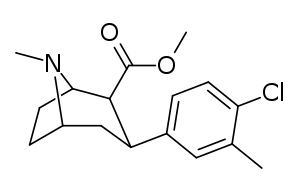
c.f. the phenyltropane class of compounds when the tropane is drawn in a manner resembling other than the boat formation.
| Substance | Structure |
|---|---|
| 2-phenylmorpholine | 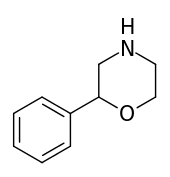 |
| 2-phenyl-3-methylmorpholine (phenmetrazine) | 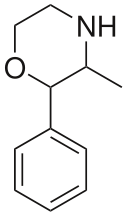 |
| 2-phenyl-3,4-dimethylmorpholine (phendimetrazine) | 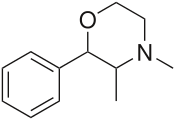 |
| 2-phenyl-3,5-dimethylmorpholine (PDM-35) | 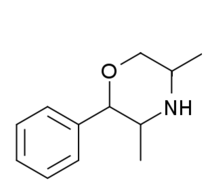 |
| 2-phenyl-3,6-dimethylmorpholine (6-methylphenmetrazine, 3,6-DMPM) | 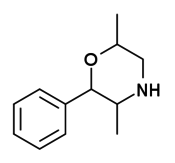 |
| 2-phenyl-5,5-dimethylmorpholine (G-130) | 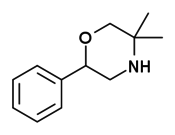 |
| 2-phenyl-3-methylmorpholin-5-one (fenmetramide) | 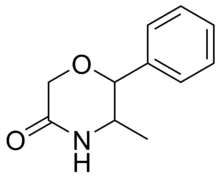 |
| 4-isopropyl-2-phenylmorpholine[2] | 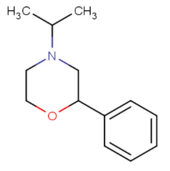 |
| 3-methyl-4-nitroso-2-phenyl-morpholine[3] | 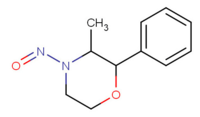 |
| 3-fluorophenmetrazine | 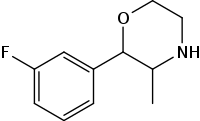 |
| 3,4-methylenedioxyphenmetrazine[4] | 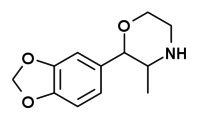 |
| 2-(2,5-dimethoxy-4-bromophenyl)morpholine[5] | 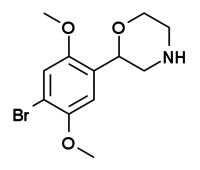 |
| 2-(3-(Trifluoromethyl)phenyl)morpholine (flumexadol)[6] | 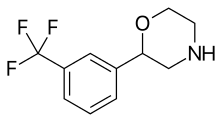 |
| Oxaflozane | 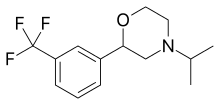 |
| 3-benzylmorpholine (3-BZM)[7] | 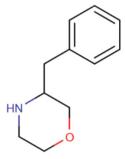 |
| (+)-(2S,3S)-2-(3-chlorophenyl)-3,5,5-trimethylmorpholin-2-ol (radafaxine) | 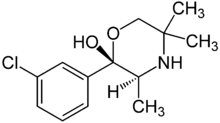 |
| (2S,3S,5R)-2-(3,5-difluorophenyl)-3,5-dimethylmorpholin-2-ol (manifaxine) | 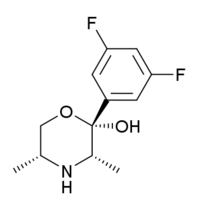 |
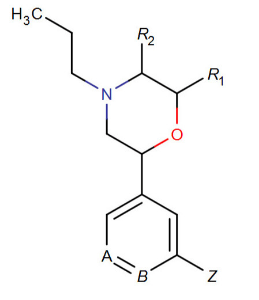
Nociceptive morpholine dopamine agonist substitution structure.[8] The cyclohexane, at where the oxygen leads to the phenyl (in-between the one and two positions on the cyclohexane itself, not the bridge of the phenyl) also have variants in this class being either a shaded or wedged bond at said oxygen. This class, however, possesses an ubiquitous propyl "tail" on the cyclohexane nitrogen compared to the DRA phenmetrazine analogue category proper.
| Substance | Structure |
|---|---|
| PF-219,061 | 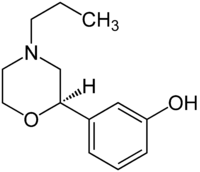 |
| PF-592,379 | 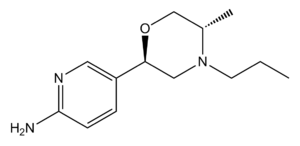 |
| OSU-6162 | 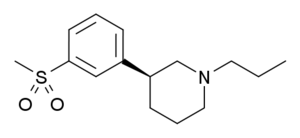 |
| 4-(4-propylmorpholin-2-yl)phenol[9] | phenol.png) |
| 2-hydroxy-5-(4-propylmorpholin-2-yl)benzamide[10] | benzamide.png) |
| (5S)-2-(3-methoxyphenyl)-5-methyl-4-propylmorpholin-2-ol[11] | -2-(3-methoxyphenyl)-5-methyl-4-propylmorpholin-2-ol.png) |
| 2-ethyl-6-(3-methoxy-phenyl)-4-propyl-morpholin-3-one[12] | -4-propyl-morpholin-3-one.png) |
See also
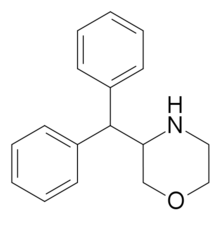
3-Benzhydrylmorpholine, a stimulant which is effectively an intermediate analog of the pipradrol class and phenmetrazine.
References
- Boswell, G. Evan (1997). "Synthesis, stereochemistry and anti-tetrabenazine activity of bicyclo analogues of 2-phenylmorpholines". Journal of Heterocyclic Chemistry. 34: 1813–1820. doi:10.1002/jhet.5570340629.
- Chem-Sink (chemicals that are Sn2 products & Friedel-Crafts alkylation reactants)
- ChemIndustry.com: 3-methyl-4-nitroso-2-phenyl-morpholine
- Świst M, Wilamowski J, Zuba D, Kochana J, Parczewski A. Determination of synthesis route of 1-(3,4-methylenedioxyphenyl)-2-propanone (MDP-2-P) based on impurity profiles of MDMA. Forensic Science International 10 May 2005, 149(2–3): 181–192. doi: 10.1016/j.forsciint.2004.06.016
- Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, McLaughlin MA, Sharif NA. Beta-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane. J Med Chem. 2004 Nov 18;47(24):6034-41. PMID 15537358
- Chemicalbook dot com: Flumexadol
- NIH PubChem Compound Summary for CID 3283983
- Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. 1st Page.
- Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. Pg. 25. Example 29 #414 diagram, schematic, and image 26
- Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. Example 48
- Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. Example 58
- Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. Example 45
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.