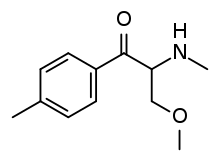
Mexedrone
Mexedrone is a stimulant drug of the cathinone class that has been sold online as a designer drug.[1][2][3][4] It is the alpha-methoxy derivative of Mephedrone.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pharmacology
Mexedrone acts as a weak serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 5289 nM, 8869 nM and 6844 nM, respectively, as well as a weak serotonin releasing agent (SRA) with an EC50 value of 2525 nM.[5]
Legal status
Mexedrone is illegal in Sweden as of 26. January 2016[6] as well as Japan as of 24. August 2016.[7]
References
- "Mexedrone". New Synthetic Drug Database.
- Qian, Zhenhua; Jia, Wei; Li, Tao; Liu, Cuimei; Hua, Zhendong (2016). "Identification and analytical characterization of four synthetic cathinone derivatives iso-4-BMC, β-TH-naphyrone, mexedrone, and 4-MDMC". Drug Testing and Analysis. 9 (2): 274–281. doi:10.1002/dta.1983. ISSN 1942-7611. PMID 27352812.
- Kuś, Piotr; Rojkiewicz, Marcin; Kusz, Joachim; Książek, Maria; Sochanik, Aleksander (14 May 2019). "Spectroscopic characterization and crystal structures of four hydrochloride cathinones: N-ethyl-2-amino-1-phenylhexan-1-one (hexen, NEH), N-methyl-2-amino-1-(4-methylphenyl)-3-methoxypropan-1-one (mexedrone), N-ethyl-2-amino-1-(3,4-methylenedioxyphenyl)pentan-1-one (ephylone) and N-butyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-chlorobutylcathinone)". Forensic Toxicology. 37 (2): 456–464. doi:10.1007/s11419-019-00477-y. ISSN 1860-8973.
- Roberts, Liam; Ford, Loretta; Patel, Neel; Vale, J. Allister; Bradberry, Sally M. (16 March 2017). "11 analytically confirmed cases of mexedrone use among polydrug users". Clinical Toxicology. 55 (3): 181–186. doi:10.1080/15563650.2016.1271424. ISSN 1556-3650. PMID 28075189.
- McLaughlin, Gavin; Morris, Noreen; Kavanagh, Pierce V.; Power, John D.; Dowling, Geraldine; Twamley, Brendan; O'Brien, John; Talbot, Brian; Walther, Donna; Partilla, John S.; Baumann, Michael H.; Brandt, Simon D. (2016). "Synthesis, characterization and monoamine transporter activity of the new psychoactive substance mexedrone and its N-methoxy positional isomer, N-methoxymephedrone". Drug Testing and Analysis. 9 (3): 358–368. doi:10.1002/dta.2053. ISSN 1942-7611. PMC 5336524. PMID 27524685.
- "31 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. November 2015.
- "指定薬物一覧" (PDF) (in Japanese). Ministry of Health, Labour and Welfare.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.