Nooglutyl
Nooglutyl is a nootropic agent that was studied at the Research Institute of Pharmacology, Russian Academy of Medical Sciences as a potential treatment for amnesia.[1]
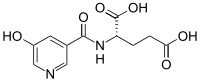 | |
| Names | |
|---|---|
| IUPAC name
N-[(5-Hydroxy-3-pyridinyl)carbonyl]-L-glutamic acid | |
| Other names
Nooglutil | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H12N2O6 |
| Molar mass | 268.225 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In animal models, it has a variety of central nervous system effects.[2][3][4][5]
References
- Flekhter, Oxana B. (2000). "Nooglutil, Russian Academy of Medical Science". Current Opinion in Central & Peripheral Nervous System Investigational Drugs. 2 (4): 491–497.
- V. V. Yasnetsov; V. A. Pravdivtsev; V. M. Popov; T. A. Voronina; N. M. Kiseleva; S. B. Kozlov (May 1995). "Antimotion Effect of Nooglutyl and Its Neuronal Mechanism". Bulletin of Experimental Biology and Medicine. 119 (5): 515–516. doi:10.1007/BF02543440. PMID 7579248. Retrieved 2011-02-08.
- Voronina, TA; Borlikova, GG; Garibova, TL; Proskuryakova, TV; Petrichenko, OB; Burd, SG; Avakyan, GN (2002). "Effect of nooglutil on benzodiazepine withdrawal syndrome and binding of 3H-spiperone with D2 receptors in rat striatum". Bulletin of experimental biology and medicine. 134 (5): 448–50. PMID 12802448.
- Garibova, TL; Galaeva, IP; Voronina, TA; Kraĭneva, VA; Kapitsa, IG; Kirichenko, SV; Makarenko, AN; Mirzoian, GR; Kuznetsova, EA (2003). "Effect of nooglutil on rats with intracerebral posttraumatic hematoma (hemorrhagic stroke)". Eksperimental'naia i klinicheskaia farmakologiia. 66 (3): 13–6. PMID 12924225.
- Povarova, OV; Garibova, TL; Kalenikova, EI; Galaeva, IP; Kraĭneva, VA; Medvedev, OS; Voronina, TA (2004). "Effect of phenyl-tert-butylnitrone, mexidol and nooglutil on the ischemic lesion zone and memory in rats following middle cerebral artery occlusion". Eksperimental'naia i klinicheskaia farmakologiia. 67 (1): 3–6. PMID 15079898.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.