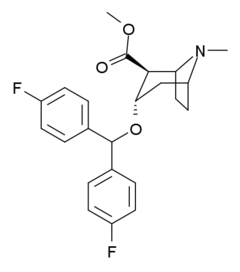
Difluoropine
Difluoropine (O-620) is a stimulant drug synthesised from tropinone, which acts as a potent and selective dopamine reuptake inhibitor. Difluoropine is unique among the tropane-derived dopamine reuptake inhibitors in that the active stereoisomer is the (S) enantiomer rather than the (R) enantiomer, the opposite way round compared to natural cocaine.[1] It is structurally related to benztropine and has similar anticholinergic and antihistamine effects in addition to its dopamine reuptake inhibitory action.[2]
 | |
| Clinical data | |
|---|---|
| Other names | (S)-(+)-2β-Carbomethoxy-3α-(bis(4-fluorophenyl)methoxy)tropane |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H25F2NO3 |
| Molar mass | 401.448 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Difluoropine has some stimulant effects in animals, although it is significantly less powerful than many of the potent phenyltropane derived stimulant drugs such as WIN 35,428 and RTI-55.[3] It showed promising effects in alleviating the symptoms of Parkinson's disease in an animal model of the disorder.[4]
It is not explicitly illegal anywhere in the world as of 2008, but might be considered to be a controlled substance analogue of cocaine on the grounds of its related chemical structure, in some jurisdictions such as the United States, Canada, Australia and New Zealand.
See also
References
- Meltzer, PC; Liang, AY; Madras, BK (1994). "The discovery of an unusually selective and novel cocaine analog: difluoropine. Synthesis and inhibition of binding at cocaine recognition sites". Journal of Medicinal Chemistry. 37 (13): 2001–10. doi:10.1021/jm00039a014. PMID 8027983.
- Campbell, VC; Kopajtic, TA; Newman, AH; Katz, JL (2005). "Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs". The Journal of Pharmacology and Experimental Therapeutics. 315 (2): 631–40. doi:10.1124/jpet.105.090829. PMID 16055673.
- Katz, JL; Izenwasser, S; Kline, RH; Allen, AC; Newman, AH (1999). "Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine". The Journal of Pharmacology and Experimental Therapeutics. 288 (1): 302–15. PMID 9862785.
- Madras, BK; Fahey, MA; Goulet, M; Lin, Z; Bendor, J; Goodrich, C; Meltzer, PC; Elmaleh, DR; et al. (2006). "Dopamine transporter (DAT) inhibitors alleviate specific parkinsonian deficits in monkeys: association with DAT occupancy in vivo". The Journal of Pharmacology and Experimental Therapeutics. 319 (2): 570–85. doi:10.1124/jpet.106.105312. PMID 16885433.