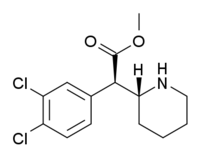
3,4-Dichloromethylphenidate
3,4-Dichloromethylphenidate (or 3,4-DCMP)[1] is a stimulant drug related to methylphenidate. Dichloromethylphenidate is a potent psychostimulant that acts as both a dopamine reuptake inhibitor and norepinephrine reuptake inhibitor, meaning it effectively boosts the levels of the norepinephrine and dopamine neurotransmitters in the brain, by binding to, and partially blocking the transporter proteins that normally remove those monoamines from the synaptic cleft.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C14H17Cl2NO2 |
| Molar mass | 302.196 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
3,4-DCMP, the threo-diastereomer, is approximately seven times more potent than methylphenidate in animal studies, but has weaker reinforcing effects due to its slower onset of action.[2][3][4][5][6] However, H. M. Deutsch's discrimination ratio implies it to be more reinforcing than cocaine.[4]
Legality
As of October 2015 3,4-CTMP is a controlled substance in China.[7]
3,4-CTMP was banned in the UK as a Temporary Class Drug from April 2015 following its unapproved sale as a designer drug.[8]
Sweden's public health agency suggested to classify 3,4-CTMP as hazardous substance on 10 November 2014.[9]
See also
References
- https://www.kingsleynapley.co.uk/insights/blogs/criminal-law-blog/temporary-class-drug-order-legal-highs-bubble-to-be-burst
- Deutsch, H.; Shi, Q.; Gruszecka-Kowalik, E.; Schweri, M. (1996). "Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs". Journal of Medicinal Chemistry. 39 (6): 1201–1209. doi:10.1021/jm950697c. PMID 8632426.
- Wayment, HK; Deutsch, H; Schweri, MM; Schenk, JO (1999). "Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists?". Journal of Neurochemistry. 72 (3): 1266–74. doi:10.1046/j.1471-4159.1999.0721266.x. PMID 10037500.
- Schweri, MM; Deutsch, HM; Massey, AT; Holtzman, SG (2002). "Biochemical and behavioral characterization of novel methylphenidate analogs". The Journal of Pharmacology and Experimental Therapeutics. 301 (2): 527–35. doi:10.1124/jpet.301.2.527. PMID 11961053.
- Davies, HM; Hopper, DW; Hansen, T; Liu, Q; Childers, SR (2004). "Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1799–802. doi:10.1016/j.bmcl.2003.12.097. PMID 15026075.
- Kim, D.; Deutsch, H.; Ye, X.; Schweri, M. (2007). "Synthesis and pharmacology of site-specific cocaine abuse treatment agents: restricted rotation analogues of methylphenidate". Journal of Medicinal Chemistry. 50 (11): 2718–2731. doi:10.1021/jm061354p. PMID 17489581.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- Methylphenidate-based NPS: A review of the evidence of use and harm. Advisory Council on the Misuse of Drugs, 31 March 2015
- "Cannabinoider föreslås bli klassade som hälsofarlig vara". Retrieved 29 June 2015.