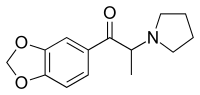
3',4'-Methylenedioxy-α-pyrrolidinopropiophenone
3',4'-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) is a stimulant designer drug. It was sold in Germany in the late 1990s and early 2000s as an ingredient in imitation ecstasy (MDMA) pills.[1] It shares a similar chemical structure with α-PPP and MDPV,[2][3][4] and has been shown to have reinforcing effects in rats.[5]
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, insufflation, Vaporization, IV, rectal, sublingual |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Primarily Urine (Renal) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C14H17NO3 |
| Molar mass | 247.28 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Legal Status
As of October 2015 MDPPP is a controlled substance in China.[6]
See also
- α-Pyrrolidinopropiophenone (α-PPP)
- 4'-Methyl-α-pyrrolidinopropiophenone (MPPP)
- 4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP)
- 3',4'-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)
References
- Springer, D.; Fritschi, G.; Maurer, H. H. (2003). "Metabolism and toxicological detection of the new designer drug 3′,4′-methylenedioxy-α-pyrrolidinopropiophenone studied in urine using gas chromatography–mass spectrometry". Journal of Chromatography B. 793 (2): 377. doi:10.1016/S1570-0232(03)00350-7.
- Maurer, HH; Kraemer, T; Springer, D; Staack, RF (2004). "Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis". Therapeutic drug monitoring. 26 (2): 127–31. doi:10.1097/00007691-200404000-00007. PMID 15228152.
- Staack, RF; Maurer, HH (2005). "Metabolism of designer drugs of abuse". Current Drug Metabolism. 6 (3): 259–74. doi:10.2174/1389200054021825. PMID 15975043.
- Springer, D; Staack, RF; Paul, LD; Kraemer, T; Maurer, HH (2005). "Identification of cytochrome P450 enzymes involved in the metabolism of 3',4'-methylenedioxy-alpha-pyrrolidinopropiophenone (MDPPP), a designer drug, in human liver microsomes". Xenobiotica. 35 (3): 227–37. doi:10.1080/00498250400028239. PMID 16019948.
- Gannon, BM; Galindo, KI; Mesmin, MP; Sulima, A; Rice, KC; Collins, GT (12 August 2017). "Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats". Neuropharmacology. doi:10.1016/j.neuropharm.2017.08.018. PMC 5809320. PMID 28811192.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.