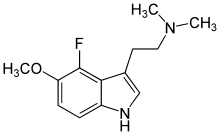
4-Fluoro-5-methoxy-DMT
4-Fluoro-5-Methoxy-N,N-dimethyltryptamine (4-F-5-MeO-DMT) was first described by David E. Nichols team in 2000. It is a potent 5-HT1A agonist. Substitution with the 4-fluorine markedly increased 5-HT1A selectivity over 5-HT2A/2C receptors with potency greater than that of the 5-HT1A agonist 8-OH-DPAT.[1]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C13H17FN2O |
| Molar mass | 236.13 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
The analog compound with the N,N-dialkyl substituents constrained into a pyrrolidine ring, is a slightly stronger agonist for the 5-HT1A receptor and retains the selectivity over the 5-HT2A/2C receptors.[2]
See also
References
- Joseph B. Blair; Deborah Kurrasch-Orbaugh; Danuta Marona-Lewicka; Medhane G. Cumbay; Val J. Watts; Eric L. Barker & David E. Nichols (2000). "Effect of Ring Fluorination on the Pharmacology of Hallucinogenic Tryptamines". Journal of Medicinal Chemistry. 43 (24): 4701–4710. doi:10.1021/jm000339w. PMID 11101361.
- Uros Laban; Deborah Kurrasch-Orbaugh; Danuta Marona-Lewicka & David E. Nichols (2001). "A Novel Fluorinated Tryptamine with Highly Potent Serotonin 5-HT1A Receptor Agonist Properties". Bioorganic & Medicinal Chemistry Letters. 11 (6): 793–795. doi:10.1016/S0960-894X(01)00062-2. PMID 11277522.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
|
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.