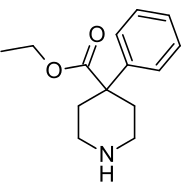
Norpethidine
Norpethidine (normeperidine, pethidine intermediate B) is a 4-phenylpiperidine derivative that is both a precursor to, and the toxic metabolite of, pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9233. The 2014 annual manufacturing quota was 11 grams (0.39 oz). [1]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | N/A |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.918 |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Norpethidine is a controlled drug because of its potential uses in manufacturing both pethidine itself and a range of N-substituted derivatives, but it has little opioid activity in its own right. Instead, norpethidine acts as a stimulant and causes convulsions.[2][3]
Build up of norpethidine is a major complication when pethidine is used in medicine as an analgesic, as when pethidine is used in high doses[4] or administered by intravenous infusion,[5] norpethidine can accumulate in the body at a faster rate than it is being excreted, particularly in elderly patients[6] or those with compromised liver or kidney function,[7] resulting in a range of toxic effects, mainly convulsions, but also myoclonus[8] and hyponatremia.[9] These complications can be serious and have sometimes resulted in death.[10]
Metabolism of pethidine to norpethidine is carried out mainly by the CYP enzymes, CYP2B6, CYP2C19 and CYP3A4, in the liver, and since the activity of these enzymes can vary between individuals and can be influenced by concurrent use of other drugs, the rate and extent of norpethidine production can be difficult to predict.[11][12]
Norpethidine can be used as a precursor in synthesis of other drugs, including etoxeridine,[13] benzethidine,[14] furethidine,[15] morpheridine, anileridine, phenoperidine, piminodine and oxpheneridine.
See also
- Nortilidine
- O-Desmethyltramadol
- Moramide intermediate
- Methadone intermediate
- Pethidine intermediate A
- Pethidine intermediate C (pethidinic acid)
References
- http://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html
- Umans JG, Inturrisi CE. Antinociceptive activity and toxicity of meperidine and normeperidine in mice. Journal of Pharmacology and Experimental Therapeutics. 1982 Oct;223(1):203-6.
- Plummer JL, Gourlay GK, Cmielewski PL, Odontiadis J, Harvey I. Behavioural effects of norpethidine, a metabolite of pethidine, in rats. Toxicology. 1995 Jan 6;95(1-3):37-44.
- Simopoulos TT, Smith HS, Peeters-Asdourian C, Stevens DS. Use of meperidine in patient-controlled analgesia and the development of a normeperidine toxic reaction. Archives of Surgery. 2002 Jan;137(1):84-8.
- Stone PA, Macintyre PE, Jarvis DA. Norpethidine toxicity and patient controlled analgesia. British Journal of Anaesthesia. 1993 Nov;71(5):738-40.
- Holmberg L, Odar-Cederlof I, Boreus LO, Heyner L, Ehrnebo M. Comparative disposition of pethidine and norpethidine in old and young patients. European Journal of Clinical Pharmacology. 1982;22(2):175-9.
- Pond SM, Tong T, Benowitz NL, Jacob P, Rigod J. Presystemic metabolism of meperidine to normeperidine in normal and cirrhotic subjects. Clinical Pharmacology and Therapeutics. 1981 Aug;30(2):183-8.
- Reutens DC, Stewart-Wynne EG. Norpethidine induced myoclonus in a patient with renal failure. Journal of Neurolology, Neurosurgery, and Psychiatry. 1989 Dec;52(12):1450-1.
- Appel WC. Possible roles of normeperidine and hyponatremia in a postoperative death. Canadian Medical Association Journal. 1987 Nov 15;137(10):912-3.
- Jiraki K. Lethal effects of normeperidine. American Journal of Forensic Medicine and Pathology. 1992 Mar;13(1):42-3.
- Ramirez J, Innocenti F, Schuetz EG, Flockhart DA, Relling MV, Santucci R, Ratain MJ. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metabolism and Disposition. 2004 Sep;32(9):930-6.
- McHugh GJ. Norpethidine accumulation and generalized seizure during pethidine patient-controlled analgesia. Anaesthesia and Intensive Care. 1999 Jun;27(3):289-91.
- U.S. Patent 2,858,316
- Frearson, P.M. et al, J. Chem. Soc., 1958, 3065-3067
- Frearson, P.M. et al, J. Chem. Soc., 1960, 2103-2107