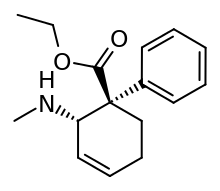
Nortilidine
Nortilidine[1] is the major active metabolite of tilidine. It is formed from tilidine by demethylation in the liver. The racemate has opioid analgesic effects roughly equivalent in potency to that of morphine[2] but virtually all of the opioid activity resides in the (1S,2R) isomer.[3] The (1R,2S) isomer has NMDA antagonist activity. The drug also acts as a dopamine reuptake inhibitor.[4] The reversed-ester of nortilidine is also known[5] which has almost identical properties to nortilidine.[6]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.3434 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- O-Desmethyltramadol, another opioid metabolite with additional (non-opioid) mechanisms of analgesia
References
- US Patent 3792080 - Process for Substituted Cyclohexenes its Products
- J Clin Pharmacol. 2002 Nov ;42 (11):1257-61 - Sequential first-pass metabolism of nortilidine: the active metabolite of the synthetic opioid drug tilidine
- Opiates: George R. Lenz page 439, Table 9-30 (78)
- Schifano, Fabrizio; Orsolini, Laura; Duccio Papanti, G.; Corkery, John M. (2015). "Novel psychoactive substances of interest for psychiatry". World Psychiatry. 14 (1): 15–26. doi:10.1002/wps.20174. ISSN 1723-8617. PMC 4329884.
- US Patent 4291059 - Cycloaromatic compounds, analgesic Properties thereof and Method of use thereof as analgesic
- Personal Communication with Derek P. Reynolds
Glutamate receptor modulators | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||
Opioid receptor modulators | |
|---|---|
| MOR |
|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted |
|
| Others |
|
See also: Receptor/signaling modulators • Signaling peptide/protein receptor modulators | |
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.