Mavoglurant
Mavoglurant (developmental code name AFQ-056) is an experimental drug candidate for the treatment of fragile X syndrome and other conditions which has been discontinued.[1][2] It exerts its effect as an antagonist of the metabotropic glutamate receptor 5 (mGluR5).[3]
 | |
| Names | |
|---|---|
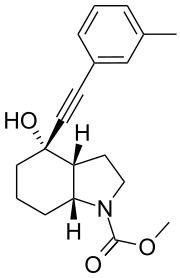
| IUPAC name
Methyl (3aR,4S,7aR)-4-hydroxy-4-[(3-methylphenyl)ethynyl]octahydro-1H-indole-1-carboxylate | |
| Other names
AFQ056 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.219.728 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H23NO3 |
| Molar mass | 313.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mavoglurant was under development by Novartis and reached phase II and phase III clinical trials.[2][4] Phase IIb/III dose finding and evaluation trials for fragile X-syndrome were discontinued by the end of 2014.[5] Otherwise, it would have been the first drug to treat the underlying disorder instead of the symptoms of fragile X syndrome.[6] Mavoglurant was also in phase II clinical trials for Levodopa-induced dyskinesia.[7][8] In 2007, Norvartis had conducted a clinical study to assess its ability of reducing cigarette smoking, but no results had been published up till now.[9] Novartis was conducting a clinical trial with this drug on obsessive–compulsive disorder.[10]
Novartis discontinued development of mavoglurant for fragile X syndrome in April 2014 following disappointing trial results.[11] Development was discontinued for other indications by 2017.[1]
See also
References
- "Mavoglurant - AdisInsight".
- P. Cole (2012). "Mavoglurant". Drugs of the Future. 37 (1): 7–12. doi:10.1358/dof.2012.37.1.1772147 (inactive 2019-08-20).
- Levenga, J; Hayashi, S; De Vrij, FM; Koekkoek, SK; Van Der Linde, HC; Nieuwenhuizen, I; Song, C; Buijsen, RA; et al. (2011). "AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome". Neurobiology of Disease. 42 (3): 311–7. doi:10.1016/j.nbd.2011.01.022. PMID 21316452.
- Jacquemont, S.; Curie, A.; Des Portes, V.; Torrioli, M. G.; Berry-Kravis, E.; Hagerman, R. J.; Ramos, F. J.; Cornish, K.; et al. (2011). "Epigenetic Modification of the FMR1 Gene in Fragile X Syndrome is Associated with Differential Response to the mGluR5 Antagonist AFQ056". Science Translational Medicine. 3 (64): 64ra1. doi:10.1126/scitranslmed.3001708. PMID 21209411.
- "Novartis Discontinues Development of mavoglurant (AFQ056) for Fragile X Syndrome • Fragile X Research - FRAXA Research Foundation". 2014-04-24.
- "AFQ056 drug improves symptoms in Fragile X patients: Study". news-medical.net. January 9, 2011.
- Kumar, R; Hauser, R. A.; Mostillo, J; Dronamraju, N; Graf, A; Merschhemke, M; Kenney, C (Sep 2013). "Mavoglurant (AFQ056) in combination with increased levodopa dosages in Parkinson's disease patients". Int J Neurosci. 126 (1): 20–4. doi:10.3109/00207454.2013.841685. PMID 24007304.
- "NHI Clinical trials".
- "Effects of AFQ056 and Nicotine in Reducing Cigarette Smoking".
- "Study to Evaluate the Effect of AFQ056 in Obsessive Compulsive Disorder (OCD)".
- FRAXA (2014). "Novartis Discontinues Development of mavoglurant (AFQ056) for Fragile X Syndrome".