Raseglurant
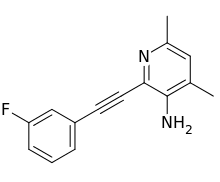
Raseglurant (INN) (code name ADX-10059) is a negative allosteric modulator of the mGlu5 receptor and derivative of MPEP which was under development by Addex Therapeutics for the treatment of migraine, gastroesophageal reflux disease, and dental anxiety.[1][2][3] It reached phase II clinical trials for all of the aforementioned indications before being discontinued due to the observation of possible predictive signs of hepatotoxicity in patients with long-term use.[3][4][5]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H13FN2 |
| Molar mass | 240.276 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Annual Reports in Medicinal Chemistry. Academic Press. 31 December 2012. pp. 80–. ISBN 978-0-12-397214-9.
- "Don't Dodge the Dentist – Tips for Dealing with Dental Anxiety". Retrieved 2017-04-05.
- Murray B. Stein; Thomas Steckler (30 July 2010). Behavioral Neurobiology of Anxiety and Its Treatment. Springer Science & Business Media. pp. 397–. ISBN 978-3-642-02912-7.
- Celia Dominguez (18 November 2010). Neurodegenerative Diseases. Springer Science & Business Media. pp. 120–. ISBN 978-3-642-16758-4.
- Nicholas J. Shaheen (25 March 2013). Benign and Neoplastic Conditions of the Esophagus, An Issue of Gastroenterology Clinics,. Elsevier Health Sciences. pp. 119–. ISBN 1-4557-7175-9.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.