Eglumegad
Eglumegad (LY354740) is a research drug developed by Eli Lilly and Company, which is being investigated for its potential in the treatment of anxiety[1] and drug addiction.[2] It is a glutamate derived compound and its mode of action implies a novel mechanism.[3]
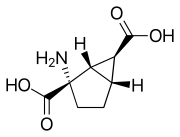 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C8H11NO4 |
| Molar mass | 185.18 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mechanism of action
Eglumegad acts as a group-selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).[4][5] It is unclear whether eglumegad directly interacts with dopamine D2 receptors.[6][7]
Effects
In experiments on mice, eglumegad was found to be as effective as diazepam for treating anxiety symptoms in several standard tests, but without producing any of the negative side effects of diazepam such as sedation and memory impairment.[8] Tests in humans confirmed that it produced anxiolytic effects without producing sedation.[9][10] However it did slightly reduce cognitive performance in tests on monkeys.[11]
Eglumegad has also been found to be effective in relieving the symptoms of withdrawal from chronic use of both nicotine[12] and morphine in animals,[13] as well as inhibiting the development of tolerance to morphine,[14] raising hope that this drug may be useful for treating drug addiction in humans.
Eglumegad and related drugs are neuroprotective[15] and are synergistic with the neuroprotection produced by N-methyl-D-aspartic acid (NMDA) antagonist drugs,[16] which may make these drugs useful in aiding recovery from brain injury.
This class of drugs also interacts with hallucinogenic drugs, with eglumegad reducing the effects of 5HT2A agonist hallucinogens,[17] while conversely the mGluR2/3 antagonist LY341495 increased the behavioural effects of these drugs.[18] This suggests that mGluR2/3 agonists such as eglumegad may have potential uses in the treatment of some forms of psychosis, although eglumegad had only limited effects on the action of the dissociative drug phencyclidine[19] which is generally a better model for schizophrenia than the 5HT2A agonist hallucinogens.[20]
Eglumegad also interferes in the hypothalamic–pituitary–adrenal axis, with chronic oral administration of this drug leading to markedly reduced baseline cortisol levels in bonnet macaques (Macaca radiata); acute infusion of eglumegad resulted in a marked diminution of yohimbine-induced stress response in those animals.[21]
In human adrenocortical cells, eglumegad has been shown to down-regulate intracellular cyclic AMP (cAMP) and steroidogenesis, with a significant decrease in aldosterone and cortisol production.[22]
Clinical development
Development of this drug and related compounds is continuing, with several clinical trials completed and more planned. Poor oral bioavailability of the original formulation led to limited efficacy in the initial human trials,[23] and so the prodrug form LY544344 did seem to be a more likely drug candidate for further development.[24][25][26][27] However a clinical trial of LY544344 was discontinued early based on findings of convulsions in preclinical studies.[28]
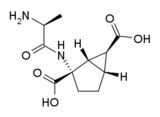
See also
References
- Pilc A (January 2003). "LY-354740 (Eli Lilly)". IDrugs. 6 (1): 66–71. PMID 12789623.
- Kłodzińska A, Chojnacka-Wójcik E, Pałucha A, Brański P, Popik P, Pilc A (December 1999). "Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models". Neuropharmacology. 38 (12): 1831–9. doi:10.1016/S0028-3908(99)00066-0. PMID 10608278.
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD (February 1997). "Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties". J. Med. Chem. 40 (4): 528–37. doi:10.1021/jm9606756. PMID 9046344.
- Schoepp DD, Johnson BG, Wright RA, Salhoff CR, Mayne NG, Wu S, Cockerman SL, Burnett JP, Belegaje R, Bleakman D, Monn JA (January 1997). "LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors". Neuropharmacology. 36 (1): 1–11. doi:10.1016/S0028-3908(96)00160-8. PMID 9144636.
- Bond A, Monn JA, Lodge D (April 1997). "A novel orally active group 2 metabotropic glutamate receptor agonist: LY354740". NeuroReport. 8 (6): 1463–6. doi:10.1097/00001756-199704140-00027. PMID 9172154.
- Seeman P, Caruso C, Lasaga M (February 2008). "Dopamine partial agonist actions of the glutamate receptor agonists LY 354,740 and LY 379,268". Synapse. 62 (2): 154–8. doi:10.1002/syn.20482. PMID 18000815.
- Fell MJ, Perry KW, Falcone JF, Johnson BG, Barth VN, Rash KS, Lucaites VL, Threlkeld PG, Monn JA, McKinzie DL, Marek GJ, Svensson KA, Nelson DL (December 2009). "In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-bicaroxylate monohydrate (LY354740) and (−)-2-oxa-4-aminobicyclo[3.1.0] Hexane-4,6-dicarboxylic acid (LY379268)". J. Pharmacol. Exp. Ther. 331 (3): 1126–36. doi:10.1124/jpet.109.160598. PMID 19755662.
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ (February 1998). "Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors". J. Pharmacol. Exp. Ther. 284 (2): 651–60. PMID 9454811.
- Grillon C, Cordova J, Levine LR, Morgan CA (August 2003). "Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans". Psychopharmacology. 168 (4): 446–54. doi:10.1007/s00213-003-1444-8. PMID 12709777.
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ (September 2003). "LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress". Stress. 6 (3): 189–97. doi:10.1080/1025389031000146773. PMID 13129812.
- Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR (April 2005). "Effects of the mGluR2/3 agonist LY354740 on computerized tasks of attention and working memory in marmoset monkeys" (PDF). Psychopharmacology. 179 (1): 292–302. doi:10.1007/s00213-004-2126-x. PMID 15678362.
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ (1997). "LY354740: a metabotropic glutamate receptor agonist which ameliorates symptoms of nicotine withdrawal in rats". Neuropharmacology. 36 (11–12): 1511–6. doi:10.1016/S0028-3908(97)00170-6. PMID 9517421.
- Vandergriff J, Rasmussen K (February 1999). "The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal". Neuropharmacology. 38 (2): 217–22. doi:10.1016/S0028-3908(98)00196-8. PMID 10218862.
- Popik P, Kozela E, Pilc A (July 2000). "Selective agonist of group II glutamate metabotropic receptors, LY354740, inhibits tolerance to analgesic effects of morphine in mice". Br. J. Pharmacol. 130 (6): 1425–31. doi:10.1038/sj.bjp.0703438. PMC 1572198. PMID 10903986.
- Kingston AE, O'Neill MJ, Lam A, Bales KR, Monn JA, Schoepp DD (July 1999). "Neuroprotection by metabotropic glutamate receptor glutamate receptor agonists: LY354740, LY379268 and LY389795". Eur. J. Pharmacol. 377 (2–3): 155–65. doi:10.1016/S0014-2999(99)00397-0. PMID 10456425.
- Allen JW, Ivanova SA, Fan L, Espey MG, Basile AS, Faden AI (July 1999). "Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury". J. Pharmacol. Exp. Ther. 290 (1): 112–20. PMID 10381766.
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (January 2000). "Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex". J. Pharmacol. Exp. Ther. 292 (1): 76–87. PMID 10604933.
- Gewirtz JC, Marek GJ (November 2000). "Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors". Neuropsychopharmacology. 23 (5): 569–76. doi:10.1016/S0893-133X(00)00136-6. PMID 11027922.
- Schreiber R, Lowe D, Voerste A, De Vry J (January 2000). "LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine". Eur. J. Pharmacol. 388 (2): R3–4. doi:10.1016/S0014-2999(99)00844-4. PMID 10666513.
- DD DD, Marek GJ (April 2002). "Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia?". Curr Drug Targets CNS Neurol Disord. 1 (2): 215–25. doi:10.2174/1568007024606177. PMID 12769628.
- Coplan JD, Mathew SJ, Smith EL, Trost RC, Scharf BA, Martinez J, Gorman JM, Monn JA, Schoepp DD, Rosenblum LA (July 2001). "Effects of LY354740, a novel glutamatergic metabotropic agonist, on nonhuman primate hypothalamic-pituitary-adrenal axis and noradrenergic function". CNS Spectr. 6 (7): 607–12, 617. doi:10.1017/S1092852900002157. PMID 15573025.
- Felizola SJA, Nakamura Y, Satoh F, Morimoto R, Kikuchi K, Nakamura T, Hozawa A, Wang L, Onodera Y, Ise K, McNamara KM, Midorikawa S, Suzuki S, Sasano H (January 2014). "Glutamate Receptors and the Regulation of Steroidogenesis in the Human Adrenal Gland: The Metabotropic Pathway". Molecular and Cellular Endocrinology. 382 (1): 170–7. doi:10.1016/j.mce.2013.09.025. PMID 24080311.
- Bergink V, Westenberg HG (November 2005). "Metabotropic glutamate II receptor agonists in panic disorder: a double blind clinical trial with LY354740". Int Clin Psychopharmacol. 20 (6): 291–3. doi:10.1097/00004850-200511000-00001. PMID 16192835.
- Bueno AB, Collado I, de Dios A, Domínguez C, Martín JA, Martín LM, Martínez-Grau MA, Montero C, Pedregal C, Catlow J, Coffey DS, Clay MP, Dantzig AH, Lindstrom T, Monn JA, Jiang H, Schoepp DD, Stratford RE, Tabas LB, Tizzano JP, Wright RA, Herin MF (August 2005). "Dipeptides as effective prodrugs of the unnatural amino acid (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740), a selective group II metabotropic glutamate receptor agonist". J. Med. Chem. 48 (16): 5305–20. doi:10.1021/jm050235r. PMID 16078848.
- Rorick-Kehn LM, Perkins EJ, Knitowski KM, Hart JC, Johnson BG, Schoepp DD, McKinzie DL (February 2006). "Improved bioavailability of the mGlu2/3 receptor agonist LY354740 using a prodrug strategy: in vivo pharmacology of LY544344". J. Pharmacol. Exp. Ther. 316 (2): 905–13. doi:10.1124/jpet.105.091926. PMID 16223873.
- Danysz W (September 2005). "LY-544344. Eli Lilly". IDrugs. 8 (9): 755–62. PMID 16118698.
- Perkins EJ, Abraham T (October 2007). "Pharmacokinetics, metabolism, and excretion of the intestinal peptide transporter 1 (SLC15A1)-targeted prodrug (1S,2S,5R,6S)-2-[(2'S)-(2-amino)propionyl]aminobicyclo[3.1.0.]hexen-2,6-dicarboxylic acid (LY544344) in rats and dogs: assessment of first-pass bioactivation and dose linearity". Drug Metab. Dispos. 35 (10): 1903–9. doi:10.1124/dmd.107.016154. PMID 17646281.
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD (June 2008). "Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder". Neuropsychopharmacology. 33 (7): 1603–10. doi:10.1038/sj.npp.1301531. PMID 17712352.