Bupropion/dextromethorphan
Bupropion/dextromethorphan (developmental code name AXS-05) is a combination drug of bupropion (Wellbutrin), a norepinephrine-dopamine reuptake inhibitor (NDRI) and nicotinic acetylcholine receptor (nAChR) antagonist, and dextromethorphan (DXM), a sigma-1 receptor agonist, NMDA receptor antagonist, and serotonin-norepinephrine reuptake inhibitor (SNRI), which is under development by Axsome Therapeutics for the treatment of treatment-resistant major depressive disorder (MDD) and agitation in Alzheimer's disease.[1][2][3] Via inhibition of CYP2D6, similarly to quinidine, bupropion slows the metabolism of DXM and thereby increases its levels. As of 2017, the combination is in phase III clinical trials for the aforementioned indications.[1]
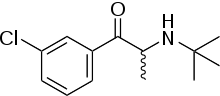 Bupropion | |
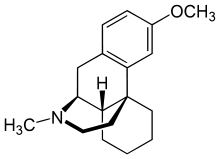 Dextromethorphan | |
| Combination of | |
|---|---|
| Bupropion | Norepinephrine reuptake inhibitor and nicotinic acetylcholine receptor antagonist |
| Dextromethorphan | Sigma-1 receptor agonist, NMDA receptor antagonist, serotonin-norepinephrine reuptake inhibitor |
| Clinical data | |
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
References
- https://adisinsight.springer.com/drugs/800044221
- Wilkinson, Samuel T.; Sanacora, Gerard (February 2019). "A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems". Drug Discovery Today. 24 (2): 606–615. doi:10.1016/j.drudis.2018.11.007. ISSN 1359-6446. PMID 30447328.
- "Axsome depression drug meets late-stage study goal, shares soar 56%". Reuters. 16 December 2019 – via www.reuters.com.
External links
Acetylcholine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Glutamate receptor modulators | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||
| DAT (DRIs) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NET (NRIs) |
| ||||||||||||||
| SERT (SRIs) |
| ||||||||||||||
| VMATs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
Sigma receptor modulators | |
|---|---|
| σ1 |
|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.