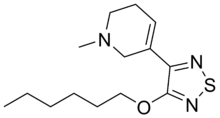
Xanomeline
Xanomeline (LY-246,708; Lumeron, Memcor) is a muscarinic acetylcholine receptor agonist with reasonable selectivity for the M1 and M4 subtypes,[1][2][3][4] though it is also known to act as a M5 receptor antagonist.[5] It has been studied for the treatment of both Alzheimer's disease and schizophrenia, particularly the cognitive and negative symptoms,[6] although gastrointestinal side effects led to a high drop-out rate in clinical trials.[7][8] Despite this, xanomeline has been shown to have reasonable efficacy for the treatment of schizophrenia symptoms, and one recent human study found robust improvements in verbal learning and short-term memory associated with xanomeline treatment.[9]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.938 |
| Chemical and physical data | |
| Formula | C14H23N3OS |
| Molar mass | 281.42 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
See also
References
- Farde L, Suhara T, Halldin C, et al. (1996). "PET study of the M1-agonists [11C]xanomeline and [11C]butylthio-TZTP in monkey and man". Dementia (Basel, Switzerland). 7 (4): 187–95. PMID 8835881.
- Jakubík J, Michal P, Machová E, Dolezal V (2008). "Importance and prospects for design of selective muscarinic agonists" (PDF). Physiological Research. 57 Suppl 3: S39–47. PMID 18481916.
- Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA (January 2009). "Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice". European Journal of Pharmacology. 603 (1–3): 147–9. doi:10.1016/j.ejphar.2008.12.020. PMID 19111716.
- Heinrich JN, Butera JA, Carrick T, et al. (March 2009). "Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists". European Journal of Pharmacology. 605 (1–3): 53–6. doi:10.1016/j.ejphar.2008.12.044. PMID 19168056.
- Grant MK, El-Fakahany EE (October 2005). "Persistent binding and functional antagonism by xanomeline at the muscarinic M5 receptor". The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 313–9. doi:10.1124/jpet.105.090134. PMID 16002459.
- Lieberman JA, Javitch JA, Moore H (August 2008). "Cholinergic agonists as novel treatments for schizophrenia: the promise of rational drug development for psychiatry". The American Journal of Psychiatry. 165 (8): 931–6. doi:10.1176/appi.ajp.2008.08050769. PMID 18676593.
- Messer WS (2002). "The utility of muscarinic agonists in the treatment of Alzheimer's disease". Journal of Molecular Neuroscience. 19 (1–2): 187–93. doi:10.1007/s12031-002-0031-5. PMID 12212779.
- Mirza NR, Peters D, Sparks RG (2003). "Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists". CNS Drug Reviews. 9 (2): 159–86. doi:10.1111/j.1527-3458.2003.tb00247.x. PMC 6741650. PMID 12847557.
- Shekhar A, Potter WZ, Lightfoot J, et al. (August 2008). "Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia". The American Journal of Psychiatry. 165 (8): 1033–9. doi:10.1176/appi.ajp.2008.06091591. PMID 18593778.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.