Octatropine methylbromide
Octatropine methylbromide (INN) or anisotropine methylbromide (USAN), trade names Valpin, Endovalpin, Lytispasm and others,[1] is a muscarinic antagonist and antispasmodic. It was introduced to the U.S. market in 1963 as an adjunct in the treatment of peptic ulcer,[2] and promoted as being more specific to the gastrointestinal tract than other anticholinergics, although its selectivity was questioned in later studies.[3][4]
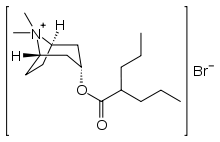 | |
| Clinical data | |
|---|---|
| Other names | 8-Methyltropinium bromide 2- propylvalerate |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | 10 to 25% (oral) |
| Protein binding | Unknown |
| Metabolism | Hepatic |
| Elimination half-life | Unknown |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.169 |
| Chemical and physical data | |
| Formula | C17H32BrNO2 |
| Molar mass | 362.345 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Octatropine has been superseded by more effective agents in the treatment of peptic ulcer disease, and is no longer used. It is still sold in some countries in combination with other drugs, such as phenobarbital and metamizole.
References
- David J. Triggle; C. R. Ganellin; F. MacDonald (1997). Dictionary of Pharmacological Agents. 2. Boca Raton: Chapman & Hall/CRC. p. 1467. ISBN 0-412-46630-9. Retrieved on August 31, 2008 through Google Book Search.
- Batterman RC, Mouratoff GJ, Kaufman JE (May 1963). "Anisotropine methylbromide: a new antispasmodic for gastrointestinal disorders". Curr Ther Res Clin Exp. 5: 213–8. PMID 13966843.
- Gyermek, Laszlo (1998). Pharmacology of antimuscarinic agents. Boca Raton: CRC Press. p. 183. ISBN 0-8493-8559-8. Retrieved on August 31, 2008 through Google Book Search.
- Bachrach WH (June 1972). "Clinical evaluation of anisotropine methyl bromide (valpin), an anticholinergic drug". Am J Dig Dis. 17 (6): 505–12. doi:10.1007/BF02231205. PMID 4555460.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.