Revefenacin
Revefenacin (trade name Yupelri) is a pharmaceutical drug for the treatment of chronic obstructive pulmonary disease (COPD). It was approved for use in the United States in 2018.[1] It was developed by Theravance Biopharma and is marketed by Mylan. Revefenacin is formulated as a solution that is nebulized and inhaled.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Yupelri |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
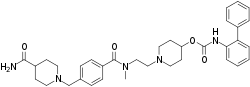
| Formula | C35H43N5O4 |
| Molar mass | 597.760 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Revefenacin is a bronchodilator that exerts its effect as a long-acting muscarinic antagonist.[3]
References
- "Theravance Biopharma and Mylan Receive FDA Approval for YUPELRI (revefenacin) in Adults with Chronic Obstructive Pulmonary Disease" (Press release). Mylan. November 9, 2018.
- Heo, Young-A (2018). "Revefenacin: First Global Approval". Drugs. doi:10.1007/s40265-018-1036-x. PMC 6445810. PMID 30560478.
- Donohue, James; Kerwin, Edward; Barnes, Chris; Moran, Edmund; Haumann, Brett; Pendyala, Srikanth; Crater, Glenn (2018). "Efficacy of Revefenacin, A Long-Acting Muscarinic Antagonist for Nebulized Therapy, in Copd Patients with Markers of More Severe Disease". Chest. 154 (4): 736A–737A. doi:10.1016/j.chest.2018.08.665.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.