Ipidacrine
Ipidacrine (Neiromidin) is a drug first synthesized by the National Research Center for Biologically Active Compounds in the Russian Federation. This compound is a ring-constricted derivative of tacrine (Cognex).[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Neiromidin |
| Other names | Amiridine; NIK-247 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.201.385 |
| Chemical and physical data | |
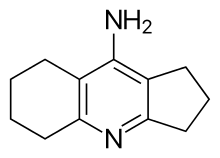
| Formula | C12H16N2 |
| Molar mass | 188.27 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ipidacrine is a reversible acetylcholinesterase inhibitor used in memory disorders of different origins.[2][3][4]
Ipidacrine directly stimulates impulse transmission in the central nervous system and neuromuscular synapses by blocking membrane potassium channels. Ipidacrine enhances not only choline, but also adrenaline, serotonin, histamine, and oxytocin effects on smooth muscle.[5]
References
- Kojima, Jun; Onodera, Kenji; Ozeki, Mitsuo; Nakayama, Kunio (1998). "Ipidacrine (NIK-247): A Review of Multiple Mechanisms as an Antidementia Agent". CNS Drug Reviews. 4 (3): 247. doi:10.1111/j.1527-3458.1998.tb00067.x.
- Damulin, IV; Stepkina, DA; Lokshina, AB (2011). "Neuromidin in mixed vascular and Alzheimer's dementia". Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova. 111 (2): 40–3. PMID 21350422.
- Pustokhanova, LV; Morozova, EM (2011). "Cognitive disorders in the early rehabilitation period of ischemic stroke and possibilities of their treatment with neuromidin". Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova. 111 (4 Pt 2): 23–7. PMID 23120773.
- Onodera, K; Kojima, J; Wachi, M (1998). "Ipidacrine (NIK-247), a novel antidementia, rapidly enters the brain and improves scopolamine-induced amnesia in rats during the Morris water maze task". Nihon shinkei seishin yakurigaku zasshi = Japanese journal of psychopharmacology. 18 (2): 33–7. PMID 9656230.
- Neiromidin Product Information
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.