Neostigmine
Neostigmine, sold under the brand name Prostigmin among others, is a medication used to treat myasthenia gravis, Ogilvie syndrome, and urinary retention without the presence of a blockage.[1][2] It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type.[1] It is given by injection either into a vein, muscle, or under the skin.[1] After injection effects are generally greatest within 30 minutes and last up to 4 hours.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prostigmin, Vagostigmin, others |
| AHFS/Drugs.com | Monograph |
| Routes of administration | IM, IV, subcutaneous, by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unclear, probably less than 5% |
| Metabolism | Slow hydrolysis by acetylcholinesterase and also by plasma esterases |
| Onset of action | Within 30 min (injection), with 4 hrs (by mouth)[1] |
| Elimination half-life | 50–90 minutes |
| Duration of action | up to 4 hrs[1] |
| Excretion | Unchanged drug (up to 70%) and alcoholic metabolite (30%) are excreted in the urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
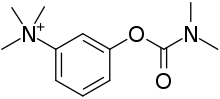
| Formula | C12H19N2O2+ |
| Molar mass | 223.296 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include nausea, increased saliva, crampy abdominal pain, and slow heart rate.[1] More severe side effects include low blood pressure, weakness, and allergic reactions.[1] It is unclear if use in pregnancy is safe for the baby.[1] Neostigmine is in the cholinergic family of medications.[1] It works by blocking the action of acetylcholinesterase and therefore increases the levels of acetylcholine.[1]
Neostigmine was patented in 1931.[3] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[4] The wholesale cost in the developing world is about US$0.18–2.6 per dose.[5] The term is from Greek neos, meaning "new", and "-stigmine", in reference to its parent molecule, physostigmine, on which it is based.[6]
Medical uses
It is used to improve muscle tone in people with myasthenia gravis, and also to reverse the effects of non-depolarizing muscle relaxants such as rocuronium and vecuronium at the end of an operation, usually in a dose of 25 to 50 μg per kilogram.
Another indication for use is the conservative management of acute colonic pseudo-obstruction, or Ogilvie's syndrome, in which patients get massive colonic dilatation in the absence of a true mechanical obstruction.[7]
Hospitals sometimes administer a solution containing neostigmine intravenously to delay the effects of envenomation through snakebite.[8] Some promising research results have also been reported for administering the drug nasally as a snakebite treatment.[9] Neostigmine was approved in 2015 in the United States to reverse the effects of muscle relaxants.[10]
Side effects
Neostigmine can induce generic ocular side effects including: headache, brow pain, blurred vision, phacodonesis, pericorneal injection, congestive iritis, various allergic reactions, and rarely, retinal detachment.[11]
Neostigmine will cause slowing of the heart rate (bradycardia); for this reason it is usually given along with a parasympatholytic drug such as atropine or glycopyrrolate.
Gastrointestinal symptoms occur earliest after ingestion and include anorexia, nausea, vomiting, abdominal cramps, and diarrhea.[12]
Pharmacology
By interfering with the breakdown of acetylcholine, neostigmine indirectly stimulates both nicotinic and muscarinic receptors. Unlike physostigmine, neostigmine has a quaternary nitrogen; hence, it is more polar and does not cross the blood–brain barrier and enter the CNS, but it does cross the placenta. Its effect on skeletal muscle is greater than that of physostigmine. Neostigmine has moderate duration of action – usually two to four hours.[13] Neostigmine binds to the anionic and ester site of cholinesterase. The drug blocks the active site of acetylcholinesterase so the enzyme can no longer break down the acetylcholine molecules before they reach the postsynaptic membrane receptors. This allows for the threshold to be reached so a new impulse can be triggered in the next neuron. In myasthenia gravis there are too few acetylcholine receptors so with the acetylcholinesterase blocked, acetylcholine can bind to the few receptors and trigger a muscular contraction.
Chemistry
Neostigmine, which can be viewed as a simplified analog of physostigmine, is made by reacting 3-dimethylaminophenol with N-dimethylcarbamoyl chloride, which forms the dimethylcarbamate, and its subsequent alkylation using dimethyl sulfate forming the desired compound.
Spectral data
Neostigmine shows notable UV/VIS absorption at 261 nm, 267 nm, and 225 nm.[14]
Neostigmine's 1H NMR Spectroscopy reveals shifts at: 7.8, 7.7, 7.4, 7.4, 3.8, and 3.1 parts per million. The higher shifts are due to the aromatic hydrogens. The lower shifts at 3.8 ppm and 3.1 ppm are due to the electronic withdrawing nature of the tertiary and quaternary nitrogen, respectively.[15]
History
Neostigmine was first synthesized by Aeschlimann and Reinert in 1931[16] and was patented by Aeschliman in 1933.[17]
Neostigmine is made by first reacting 3-dimethylaminophenol with N-dimethylcarbamoyl chloride, which forms a dimethylcarbamate. Next, that product is alkylated using dimethyl sulfate, which forms neostigmine.[18]
See also
- Miotine
- T-1123
- TL-1238
References
- "Neostigmine Bromide". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- WHO Model Formulary 2008 (PDF). World Health Organization. 2009. p. 428. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 540. ISBN 9783527607495. Archived from the original on 2016-12-20.
- "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- "Neostigmine Methylsulfate". International Drug Price Indicator Guide. Archived from the original on 10 May 2017. Retrieved 8 December 2016.
- "neostigmine: definition of neostigmine in Oxford dictionary (American English) (US)". www.oxforddictionaries.com. Archived from the original on 2015-12-22. Retrieved 2015-12-17.
- Maloney, Nell; Vargas, H. David (2005-05-01). "Acute Intestinal Pseudo-Obstruction (Ogilvie's Syndrome)". Clinics in Colon and Rectal Surgery. 18 (2): 96–101. doi:10.1055/s-2005-870890. ISSN 1531-0043. PMC 2780141. PMID 20011348.
- Franklin, Deborah, "Potential Treatment For Snakebites Leads To A Paralyzing Test Archived 2014-08-09 at the Wayback Machine", NPR.org, July 31, 2013.
- "Universal antidote for snakebite: Experimental trial represents promising step Archived 2014-07-07 at the Wayback Machine", California Academy of Sciences via Science Daily, May 28, 2014.
- "2015 Approval letter from FDA for the use of neostigmine to reverse the effects of non-depolarising muscle relaxants after surgery" (PDF). www.accessdata.fda.gov. Retrieved 2019-01-13.
- Gilman, Goodman & Gilman 1980, p. 114.
- Gilman, Goodman & Gilman 1980, p. 109.
- Howland, R.D., Mycek, M.J., Harvey, R.A., Champe, P.C., and Mycek, M.J., Pharmacology 3rd edition, Lippincott's Illustrated Reviews, 2008, p. 51.
- Porst H; Kny L (May 1985). "The structure of degradation products of neostigmine bromide". Die Pharmazie (in German). 40 (5): 325–8. PMID 4034636.
- Ferdous, Abu J; Waigh, Roger D (1993). "Application of the WATR technique for water suppression in 1H NMR spectroscopy in determination of the kinetics of hydrolysis of neostigmine bromide in aqueous solution". Journal of Pharmacy and Pharmacology. 45 (6): 559–562. doi:10.1111/j.2042-7158.1993.tb05598.x. PMID 8103105.
- Whitacre 2007, p. 57.
- Aeschliman, John A., U.S. Patent 1,905,990 (1933).
- Gilman, Goodman & Gilman 1980, p. 103.
Bibliography
- Gilman, A.G.; Goodman, L.S.; Gilman, A. (1980). Goodman & Gilman's The Pharmacological Basis of Therapeutics (6th ed.). New York: Macmillan Publishing Co., Inc.
- Whitacre, David M. (2007). Reviews of Environmental Contamination and Toxicology. Springer Science+Business Media. p. 57. ISBN 978-0-387-73162-9.