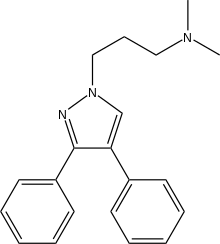
Fezolamine
Fezolamine (Win-41,528-2) is a drug which was investigated by Sterling Drug as an antidepressant in the 1980s.[1][2] The isomeric N,N-dimethyl-4,5-diphenyl-1H-pyrazole-1-propanamine was completely inactive in the primary antidepressant screens.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H23N3 |
| Molar mass | 305.42 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
It acts as a serotonin, norepinephrine, and dopamine reuptake inhibitor, with 3- to 4-fold preference for the former neurotransmitter.[3] It was found to be effective and well tolerated in clinical trials but was never marketed.[4]
See also
References
- U.S. Patent 4,182,895
- Bailey, D. M.; Hansen, P. E.; Hlavac, A. G.; Baizman, E. R.; Pearl, J.; Defelice, A. F.; Feigenson, M. E. (1985). "3,4-Diphenyl-1H-pyrazole-1-propanamine antidepressants". Journal of Medicinal Chemistry. 28 (2): 256–60. doi:10.1021/jm00380a020. PMID 3968690.
- Baizman, E. R.; Ezrin, A. M.; Ferrari, R. A.; Luttinger, D (1987). "Pharmacologic profile of fezolamine fumarate: A nontricyclic antidepressant in animal models". The Journal of Pharmacology and Experimental Therapeutics. 243 (1): 40–54. PMID 3668867.
- Zisook, S.; Mendels, J.; Janowsky, D.; Feighner, J.; Lee, J. C. M.; Fritz, A. (1987). "Efficacy and Safety of Fezolamine in Depressed Patients". Neuropsychobiology. 17 (3): 133–8. doi:10.1159/000118353. PMID 3683802.
| DAT (DRIs) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NET (NRIs) |
| ||||||||||||||
| SERT (SRIs) |
| ||||||||||||||
| VMATs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.