Amitifadine
Amitifadine (developmental code names DOV-21,947, EB-1010) is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) or so-called triple reuptake inhibitor (TRI) which is or was being developed by Euthymics Bioscience[1][2] It was under development for the treatment of major depressive disorder, but in May 2013, it was reported that the drug failed to show superior efficacy to placebo in a phase IIb/IIIa clinical trial.[3] It was suggested that this may have been due to the drug being underdosed.[3] In September 2017, development of amitifadine for the treatment of major depressive disorder was finally officially discontinued.[1] As of September 2017, it is still listed as being under development for the treatment of alcoholism and smoking withdrawal.[1]
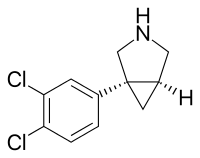 | |
| Clinical data | |
|---|---|
| Pronunciation | (/æmɪˈtɪfədiːn/ am-i-TIF-ə-deen) |
| Other names | DOV-21,947; EB-1010 |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H11Cl2N |
| Molar mass | 228.118 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pharmacology
Ki values for SERT, NET, and DAT of amitifadine are 99 nM, 262 nM, and 213 nM.[2] The IC50 values for serotonin, norepinephrine and dopamine uptake are 12, 23 and 96 nM, respectively
| Compound | Uptake (IC50, nM) | Binding (Ki, nM) | ||||
|---|---|---|---|---|---|---|
| 5-HT | NE | DA | SERT | NET | DAT | |
| Amitifadine | 12 | 23 | 96 | 100 | 260 | 210 |
| DOV-216,303 | 14 | 20 | 78 | 190 | 380 | 190 |
| DOV-102,677 | 130 | 100 | 130 | 740 | 1000 | 220 |
Amitifadine reduces the duration of immobility in the forced swim test in rats with an oral minimum effective dose (MED) of 5 mg/kg. This antidepressant-like effect manifests in the absence of significant increases in motor activity at doses of up to 20 mg/kg. Amitifadine also produces a dose-dependent reduction in immobility in the tail suspension test, with an oral MED of 5 mg/kg. In microdialysis studies, amitifadine increased extracellular levels of serotonin, norepinephrine and dopamine in brain regions and did not induce hyperactivity in rats.[4] Results in a small clinical trial indicated that amitifadine had statistically significant antidepressant effects and was well tolerated.[5]
Chemistry
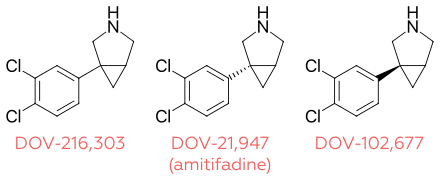
Amitifadine is the (+)-enantiomer of DOV-216,303, and its (−)-enantiomer is DOV-102,677.
References
- "Amitifadine". AdisInsight. Retrieved 26 February 2017.
- Skolnick, P.; Popik, P.; Janowsky, A.; Beer, B.; Lippa, A. S. (2003). "Antidepressant-like actions of DOV 21,947: a "triple" reuptake inhibitor". European Journal of Pharmacology. 461 (2–3): 99–104. doi:10.1016/S0014-2999(03)01310-4. PMID 12586204.
- "Archived copy" (PDF). Archived from the original (PDF) on 2017-09-24. Retrieved 2017-06-12.CS1 maint: archived copy as title (link)
- Golembiowska, K.; Kowalska, M.; Bymaster, F. P. (2012). "Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity". Synapse. 66 (5): 435–444. doi:10.1002/syn.21531. PMID 22213370.
- Tran, P.; Skolnick, P.; Czobor, P.; Huang, N. Y.; Bradshaw, M.; McKinney, A.; Fava, M. (2012). "Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial". Journal of Psychiatric Research. 46 (1): 64–71. doi:10.1016/j.jpsychires.2011.09.003. PMID 21925682.