Olopatadine
Olopatadine is a medication used to decrease the symptoms of allergic conjunctivitis and allergic rhinitis (hay fever).[1] It is used as eye drops or as a nasal spray.[1] The eye drops general result in an improvement within half an hour.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Patanol and others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602025 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Eye drops, nasal spray |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
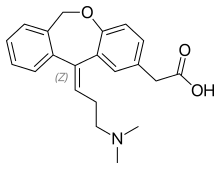
| Formula | C21H23NO3 |
| Molar mass | 337.412 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include headache, sore throat, eye discomfort, change in taste.[2][1] More significant side effects may include sleepiness.[1] It is unclear if use during pregnancy or breastfeeding is safe.[3] It is an antihistamine and mast cell stabilizer.[4][1]
Olopatadine was patented in 1986 and came into medical use in 1997.[5] It is available as a generic medication.[1] A 5 milliliter bottle of the eye drops in the United Kingdom costs the NHS less than £5 as of 2019.[2] In the United States the wholesale cost of this amount is about US$12.50.[6] In 2016 it was the 269th most prescribed medication in the United States with more than a million prescriptions.[7]
Medical uses
It is used to treat allergic conjunctivitis and hay fever.[1] It is used as eye drops and as a nasal spray.[1]
Side effects
Some known side effects include headache (7% of occurrence), eye burning and/or stinging (5%), blurred vision, dry eyes, foreign body sensation, hyperemia, keratitis, eyelid edema, pruritus, asthenia, sore throat (pharyngitis), rhinitis, sinusitis, taste perversion, and vomiting.
Chemistry
Synthesis
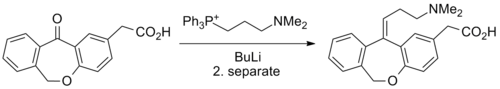
Pharmacology
Pharmacodynamics
Olopatadine acts as a selective antagonist of the histamine H1 receptor, thus stabilizing mast cells and inhibiting histamine release.
History
Olopatadine was patented in 1986 by Kyowa Hakko Kogyo and came into medical use in 1997.[5]
Brand names
Brand names include Pazeo, Pataday, Patanol S, Patanol, Opatanol, Olopat, Patanase.[9] It is also available as an oral tablet in Japan under the tradename Allelock, manufactured by Kyowa Hakko Kogyo.[10]
References
- "Olopatadine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 26 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1126. ISBN 9780857113382.
- "Olopatadine ophthalmic Use During Pregnancy". Drugs.com. Retrieved 26 March 2019.
- Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A (2015). "Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis" (PDF). Cochrane Database Syst Rev. 6 (6): CD009566. doi:10.1002/14651858.CD009566.pub2. hdl:2164/6048. PMID 26028608.CS1 maint: uses authors parameter (link)
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 549. ISBN 9783527607495.
- "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- Ueno, K.; Kubo, S.; Tagawa, H.; Yoshioka, T.; Tsukada, W.; Tsubokawa, M.; Kojima, H.; Kasahara, A. (1976). "6,11-Dihydro-11-oxodibenz[b,e]oxepinacetic acids with potent antiinflammatory activity". Journal of Medicinal Chemistry. 19 (7): 941–946. doi:10.1021/jm00229a017. PMID 940112.
- Drugs.com, Alcon's Patanase Nasal Spray Approved by FDA for Treatment of Nasal Allergy Symptoms
- Kyowa Hakko Kogyo Co., Ltd. (2007). "ALLELOCK Tablets 2.5 & ALLELOCK Tablets 5 (English)" (PDF). Retrieved 2008-08-10.