Nizatidine
Nizatidine is a histamine H2 receptor antagonist that inhibits stomach acid production, and is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease.
 | |
| Clinical data | |
|---|---|
| Trade names | Axid, Tazac |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >70% |
| Protein binding | 35% |
| Metabolism | Liver |
| Elimination half-life | 1–2 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.155.683 |
| Chemical and physical data | |
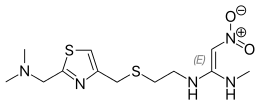
| Formula | C12H21N5O2S2 |
| Molar mass | 331.46 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was patented in 1980 and approved for medical use in 1987.[2] It was developed by Eli Lilly. Brand names include Tazac and Axid.
Medical use
Nizatidine is used to treat duodenal ulcers, gastric ulcers, and gastroesophageal reflux disease (GERD/GORD), and to prevent stress ulcers.[1]
Adverse effects
Side effects are uncommon, usually minor, and include diarrhea, constipation, fatigue, drowsiness, headache, and muscle aches.[1]
History and development
Nizatidine was developed by Eli Lilly, and was first marketed in 1987. It is considered to be equipotent with ranitidine and differs by the substitution of a thiazole ring in place of the furan ring in ranitidine. In September 2000, Eli Lilly announced they would sell the sales and marketing rights for Axid to Reliant Pharmaceuticals.[3] Subsequently, Reliant developed the oral solution of Axid, marketing this in 2004, after gaining approval from the U.S. Food and Drug Administration (FDA).[4] However, a year later, they sold rights of the Axid Oral Solution (including the issued patent[5] protecting the product) to Braintree Laboratories.[6]
Nizatidine proved to be the last new histamine H2 receptor antagonist introduced prior to the advent of proton pump inhibitors.
See also
- Famotidine (Pepcid) — another popular H2 receptor antagonist
References
- "Nizatidine". Livertox.nih.gov. Retrieved 2015-10-11.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 44. ISBN 9783527607495.
- Archived May 26, 2008, at the Wayback Machine
- Archived December 26, 2013, at the Wayback Machine
- "United States Patent: 6930119". Patft.uspto.gov. Retrieved 2015-10-11.
- Archived August 14, 2007, at the Wayback Machine