Burimamide
Burimamide is an antagonist at the H2 and H3 histamine receptors. It is largely inactive as an H2 antagonist at physiological pH,[1] but its H3 affinity is 100x higher. It is a thiourea derivative.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
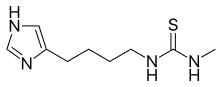
1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H16N4S |
| Molar mass | 212.32 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Burimamide was first developed by scientists at Smith, Kline & French (SK&F; now GlaxoSmithKline) in their intent to develop a histamine antagonist for the treatment of peptic ulcers.[2] The discovery of burimamide ultimately led to the development of cimetidine (Tagamet).[2]
See also
References
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. p. 205. ISBN 978-0-19-850346-0.
- "Tagamet®: Discovery of Histamine H2-receptor Antagonists". National Historic Chemical Landmarks. American Chemical Society. Archived from the original on December 9, 2012. Retrieved June 25, 2012.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.