Amthamine
Amthamine is a histamine agonist selective for the H2 subtype.[1] It has been used in vitro and in vivo to study gastric secretion,[2] as well as other functions of the H2 receptor.[3][4][5]
 | |
| Names | |
|---|---|
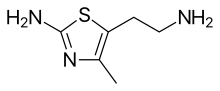
| IUPAC name
5-(2-aminoethyl)-4-methyl-1,3-thiazol-2-amine | |
| Other names
5-(2-aminoethyl)-4-methyl-2-thiazolamine 2-amino-5-(2-aminoethyl)-4-methylthiazole | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H11N3S |
| Molar mass | 157.236 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Eriks, J. C; Van Der Goot, H; Sterk, G. J; Timmerman, H (1992). "Histamine H2-receptor agonists. Synthesis, in vitro pharmacology, and qualitative structure-activity relationships of substituted 4- and 5-(2-aminoethyl)thiazoles". Journal of Medicinal Chemistry. 35 (17): 3239–46. doi:10.1021/jm00095a021. PMID 1507209.
- Coruzzi G, Timmerman H, Adami M, Bertaccini G (July 1993). "The new potent and selective histamine H2 receptor agonist amthamine as a tool to study gastric secretion". Naunyn Schmiedebergs Arch Pharmacol. 348 (1): 77–81. doi:10.1007/BF00168540. PMID 8377843.
- Ezeamuzie, C. I; Philips, E (2000). "Histamine H(2) receptors mediate the inhibitory effect of histamine on human eosinophil degranulation". British Journal of Pharmacology. 131 (3): 482–8. doi:10.1038/sj.bjp.0703556. PMC 1572337. PMID 11015298.
- Fernandez, N; Monczor, F; Baldi, A; Davio, C; Shayo, C (2008). "Histamine H2 receptor trafficking: Role of arrestin, dynamin, and clathrin in histamine H2 receptor internalization". Molecular Pharmacology. 74 (4): 1109–18. doi:10.1124/mol.108.045336. PMID 18617631.
- Threlfell, S; Exley, R; Cragg, S. J; Greenfield, S. A (2008). "Constitutive histamine H2 receptor activity regulates serotonin release in the substantia nigra". Journal of Neurochemistry. 107 (3): 745–55. doi:10.1111/j.1471-4159.2008.05646.x. PMID 18761715.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.