Acrivastine
Acrivastine is a medication used for the treatment of allergies and hay fever. It is a second-generation H1-receptor antagonist antihistamine (like its base molecule triprolidine) and works by blocking histamine H1 receptors.
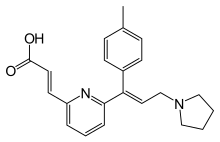 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682619 |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1.5 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.306 |
| Chemical and physical data | |
| Formula | C22H24N2O2 |
| Molar mass | 348.438 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
This non-sedating antihistamine is sold under the brand name Benadryl Allergy Relief in the United Kingdom by McNeil Laboratories. It should not be confused with Benadryl Once a Day which has cetirizine as the active ingredient and is also sold by McNeil in the UK. It is available as an over-the-counter medicine in the UK, and is available with or without pseudoephedrine under the Benadryl brand.
In the U.S., acrivastine is the active ingredient in the Semprex brand. Semprex-D also contains the decongestant pseudoephedrine. Semprex-D is marketed in the U.S. by Actient Pharmaceuticals.[1]
Comparisons with other popular antihistamines
Unlike cetirizine or loratadine, for which the standard dose is one tablet per day, a single acrivastine tablet may be taken up to three times a day.[2] It is not to be taken by people over the age of 65, pregnant women, or people with compromised liver or kidney function.
References
- SEMPREX-D - acrivastine and pseudoephedrine hydrochloride capsule U.S. National Library of Medicine, National Institutes of Health, May 2008
- "Benadryl Allergy Relief". electronic Medicines Compendium (eMC). 2014. Archived from the original on 27 September 2012. Retrieved 4 July 2014.