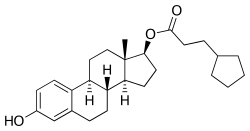
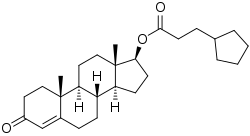
Estradiol cypionate/testosterone cypionate
Estradiol cypionate/testosterone cypionate (EC/TC), sold under the brand names Depo-Testadiol and Femovirin among others, is an injectable combination medication of estradiol cypionate (EC), an estrogen, and testosterone cypionate (TC), an androgen/anabolic steroid, which is used in menopausal hormone therapy for women.[1] It is specifically indicated for the treatment of moderate-to-severe vasomotor symptoms (i.e., hot flashes), but can also be used for other estrogen indications in women.[1] The medication has also been used to suppress lactation in postpartum women.[2]
 | |
 | |
| Combination of | |
|---|---|
| Estradiol cypionate | Estrogen |
| Testosterone cypionate | Androgen; Anabolic steroid |
| Clinical data | |
| Trade names | Depo-Testadiol, Femovirin, others |
| Other names | EC/TC |
| Routes of administration | Intramuscular injection |
Depo-Testadiol was provided in the form of 10 mL vials containing 2 mg/mL EC and 50 mg/mL TC in an oil solution and was administered by intramuscular injection once every 4 weeks.[1] Conversely, Femovirin was provided in the form of 1 mL ampoules containing 3.5 mg/mL EC (2.4 mg/mL free estradiol) and 90 mg/mL TC (62.9 mg/mL free testosterone) in an oil solution and was administered by intramuscular injection once every 4 to 6 weeks.[3][4][5][6][7] The elimination half-life of EC in oil by intramuscular injection is approximately 5 days, while the elimination half-life of TC in oil by intramuscular injection is approximately 8 days.[1] EC/TP reportedly has a duration of about 21 days.[8]
EC/TC likely poses a considerably increased risk of endometrial hyperplasia and cancer in women with intact uteruses (i.e., women who are not hysterectomized) if it is not combined with a progestogen.[1] This is due to the EC component.[1] The concomitant use of a progestogen will abolish such risks.[1] The medication can also cause masculinization, such as acne, deepened voice, hirsutism, and increased sex drive, due to its TC component.[1] Some of these masculinizing symptoms, such as voice deepening, can be irreversible.[1]
Depo-Testadiol was introduced for medical use in 1954,[9] while Femovirin was introduced for medical use in 1956.[10] An oral tablet product with the same brand name of Femovirin, containing ethinylestradiol and methyltestosterone, was marketed in 1958, and should not be confused with the injectable Femovirin.[11][4] Depo-Testadiol was discontinued in the United States by 2013.[12] Both Depo-Testadiol and Femovirin have been discontinued in most other countries, but formulations of EC/TC under other brand names continue to be marketed in Taiwan.[13][14][15]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg 1x/1–2 days |
| Methyltestosterone | Metandren, Estratest | Tablet | 0.5–10 mg/day | |
| Fluoxymesterone | Halotestin | Tablet | 1–2.5 mg 1x/1–2 days | |
| Normethandronea | Ginecoside | Tablet | 5 mg/day | |
| Tibolone | Livial | Tablet | 1.25–2.5 mg/day | |
| Prasterone (DHEA)b | – | Tablet | 10–100 mg/day | |
| Sublingual | Methyltestosterone | Metandren | Tablet | 0.25 mg/day |
| Transdermal | Testosterone | Intrinsa | Patch | 150–300 μg/day |
| AndroGel | Gel, cream | 1–10 mg/day | ||
| Vaginal | Prasterone (DHEA) | Intrarosa | Insert | 6.5 mg/day |
| Injection | Testosterone propionatea | Testoviron | Oil solution | 25 mg 1x/1–2 weeks |
| Testosterone enanthate | Delatestryl, Primodian Depot | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone cypionate | Depo-Testosterone, Depo-Testadiol | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone isobutyratea | Femandren M, Folivirin | Aqueous suspension | 25–50 mg 1x/4–6 weeks | |
| Mixed testosterone esters | Climacterona | Oil solution | 150 mg 1x/4–8 weeks | |
| Omnadren, Sustanon | Oil solution | 50–100 mg 1x/4–6 weeks | ||
| Nandrolone decanoate | Deca-Durabolin | Oil solution | 25–50 mg 1x/6–12 weeks | |
| Prasterone enanthatea | Gynodian Depot | Oil solution | 200 mg 1x/4–6 weeks | |
| Implant | Testosterone | Testopel | Pellet | 50–100 mg 1x/3–6 months |
| Notes: Premenopausal women produce about 230 ± 70 μg testosterone per day (6.4 ± 2.0 mg testosterone per 4 weeks), with a range of 130 to 330 μg per day (3.6–9.2 mg per 4 weeks). Footnotes: a = Mostly discontinued or unavailable. b = Over-the-counter. Sources: See template. | ||||
References
- "Download Limit Exceeded". citeseerx.ist.psu.edu.
- Vorherr H (July 1972). "Suppression of postpartum lactation". Postgrad Med. 52 (1): 145–52. doi:10.1080/00325481.1972.11713186. PMID 5037562.
- Fr. Kauffmann (1959). Fünfundsechzigster Kongress: Gehalten zu Wiesbaden vom 6.–9. April 1959. Springer-Verlag. pp. 162–166. ISBN 978-3-642-96026-0.
- Hans Hermann Julius Hager; Walther Kern; Paul Heinz List; Hermann Josef Roth (29 July 2013). Hagers Handbuch der Pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Ärzte und Medizinalbeamte: Wirkstoffgruppen II Chemikalien und Drogen (A-AL). Springer-Verlag. pp. 156, 185. ISBN 978-3-662-25655-8.
- A. Saure (11 November 2013). Die Wechseljahre der Frau: Hormone — Präparate — Therapien. Springer-Verlag. pp. 157–. ISBN 978-3-0348-6676-7.
- Georg Arends; Heinrich Zörnig; Hermann Hager; Georg Frerichs, Walther Kern (14 December 2013). Hagers Handbuch der pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Drogisten, Ärzte u. Medizinalbeamte. Springer-Verlag. pp. 1164–. ISBN 978-3-662-36329-4.
- E. Buchborn; H. Jahrmärker; H.J. Karl; G.A. Martini, W. Müller, G. Riecker, H. Schwiegk, W. Siegenthaler, W. Stich (2 July 2013). Therapie innerer Krankheiten. Springer-Verlag. pp. 405–. ISBN 978-3-662-10489-7.CS1 maint: multiple names: authors list (link)
- Ufer, Joachim (1 January 1978). Hormontherapie in der Frauenheilkunde: Grundlagen und Praxis [Hormone Therapy in Gynecology: Principles and Practice] (in German) (5 ed.). de Gruyter. p. 276. ISBN 978-3110066647. OCLC 924728827.
- "NEW Prescription Products". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 16 (3): 193–200. 1955. doi:10.1016/S0095-9561(16)33664-7. ISSN 0095-9561.
- "Neue Spezialitäten". Klinische Wochenschrift. 34 (29–30): 819–819. 1956. doi:10.1007/BF01468058. ISSN 0023-2173.
- "Neue Spezialitäten". Klinische Wochenschrift. 36 (24): 1169–1169. 1958. doi:10.1007/BF01481649. ISSN 0023-2173.
- Food and Drug Administration. Approved Drug Products with Therapeutic Equivalence Evaluations - FDA Orange Book 33rd Edition (2013): FDA Orange Book 33rd Edition (2013). Logos Press. pp. 619–. ISBN 978-1-934899-83-0.
- "Estradiol". Drugs.com.
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 2100, 2124–2125. ISBN 978-0-85369-840-1.
- "IBM Watson Health Products: Please Login". www.micromedexsolutions.com.