Oxymetholone
Oxymetholone, sold under the brand names Anadrol and Anapolon among others, is an androgen and anabolic steroid (AAS) medication which is used primarily in the treatment of anemia.[3][4] It is also used to treat osteoporosis, HIV/AIDS wasting syndrome, and to promote weight gain and muscle growth in certain situations.[3] It is taken by mouth.[3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Anadrol, Anapolon, others |
| Other names | CI-406; NSC-26198; 2-Hydroxymethylene-17α-methyl-4,5α-dihydrotestosterone; 2-Hydroxymethylene-17α-methyl-DHT; 2-Hydroxymethylene-17α-methyl-5α-androstan-17β-ol-3-one |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed[1] |
| Metabolism | Liver[1][2] |
| Elimination half-life | Unknown[2] |
| Excretion | Urine[1][2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.454 |
| Chemical and physical data | |
| Formula | C21H32O3 |
| Molar mass | 332.48 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Side effects of oxymetholone include increased sexual desire as well as symptoms of masculinization like acne, increased hair growth, and voice changes.[3] It can also cause liver damage.[3][4] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[3][5] It has strong anabolic effects and weak androgenic effects.[3]
Oxymetholone was first described in 1959 and was introduced for medical use by 1961.[3][6][7][8] It is used mostly in the United States.[3][9] In addition to its medical use, oxymetholone is used to improve physique and performance.[3] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[3]
Medical uses
The primary clinical applications of oxymetholone include treatment of anemia and osteoporosis, as well as stimulating muscle growth in malnourished or underdeveloped patients.[3] However, in the United States, the only remaining FDA-approved indication is the treatment of anemia.[3][10]
Following the introduction of oxymetholone, nonsteroidal drugs such as epoetin alfa were developed and shown to be more effective as a treatment for anemia and osteoporosis without the side effects of oxymetholone.[3] The drug remained available despite this and eventually found a new use in treating HIV/AIDS wasting syndrome.[3]
Presented most commonly as a 50 mg tablet, oxymetholone has been said to be one of the "strongest" and "most powerful" AAS available for medical use.[3][11] Similarly, there is a risk of side effects.[12][13] Oxymetholone is highly effective in promoting extensive gains in body mass, mostly by greatly improving protein synthesis.[3] For this reason, it is often used by bodybuilders and athletes.[3]
Non-medical uses
Oxymetholone is used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[3]
Side effects
The common side effects of oxymetholone include depression, lethargy, headache, swelling, rapid weight gain, priapism, changes in skin color, urination problems, nausea, vomiting, stomach pain (if taken on an empty stomach), loss of appetite, jaundice, breast swelling in men, feeling restless or excited, insomnia, and diarrhea.[12] In women, side effects also include acne, changes in menstrual periods, voice deepening, hair growth on the chin or chest, pattern hair loss, enlarged clitoris, and changes in libido.[3][12] Because of its 17α-alkylated structure, oxymetholone is hepatotoxic.[3] Long term use of the drug can cause a variety of serious ailments, including hepatitis, liver cancer, and cirrhosis; therefore periodic liver function tests are recommended for those taking oxymetholone.[13]
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Like other AAS, oxymetholone is an agonist of the androgen receptor (AR).[3] It is not a substrate for 5α-reductase (as it is already 5α-reduced) and is a poor substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), and therefore shows a high ratio of anabolic to androgenic activity.[3]
As a DHT derivative, oxymetholone is not a substrate for aromatase and hence cannot be aromatized into estrogenic metabolites.[3] However, uniquely among DHT derivatives, oxymetholone is nonetheless associated with relatively high estrogenicity, and is known to have the potential to produce estrogenic side effects such as gynecomastia (rarely) and water retention.[3][14][15][16] It has been suggested that this may be due to direct binding to and activation of the estrogen receptor by oxymetholone.[3] Oxymetholone does not possess any significant progestogenic activity.[3]
Pharmacokinetics
There is limited information available on the pharmacokinetics of oxymetholone.[4] It appears to be well-absorbed with oral administration.[4] Oxymetholone has very low affinity for human serum sex hormone-binding globulin (SHBG), less than 5% of that of testosterone and less than 1% of that of DHT.[1] The drug is metabolized in the liver by oxidation at the C2 position, reduction at the C3 position, hydroxylation at the C17 position, and conjugation.[4][2] The C2 hydroxymethylene group of oxymetholone can be cleaved to form mestanolone (17α-methyl-DHT), which may contribute to the effects of oxymetholone.[3] The elimination half-life of oxymetholone is unknown.[2] Oxymetholone and its metabolites are eliminated in the urine.[1][2]
Chemistry
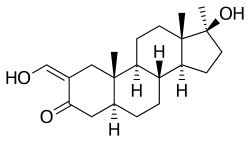
Oxymetholone, also known as 2-hydroxymethylene-17α-methyl-4,5α-dihydrotestosterone (2-hydroxymethylene-17α-methyl-DHT) or as 2-hydroxymethylene-17α-methyl-5α-androstan-17β-ol-3-one, is a synthetic androstane steroid and a 17α-alkylated derivative of DHT.[17][18][3]
History
Oxymetholone was first described in a 1959 paper by scientists from Syntex.[3][6] It was introduced for medical use by Syntex and Imperial Chemical Industries in the United Kingdom under the brand name Anapolon by 1961.[7][8] Oxymetholone was also introduced under the brand names Adroyd (Parke-Davis) by 1961 and Anadrol (Syntex) by 1962.[19][20][21] The drug was marketed in the United States in the early 1960s.[3]
Society and culture
Generic names
Oxymetholone is the generic name of the drug and its INN, USAN, USP, BAN, and JAN, while oxymétholone is its DCF.[17][18][22][9]
Brand names
Oxymetholone has been marketed under a variety of brand names including Anadrol, Anadroyd, Anapolon, Anasterona, Anasteronal, Anasterone, Androlic, Androyd, Hemogenin, Nastenon, Oxitoland, Oxitosona, Oxyanabolic, Oxybolone, Protanabol, Roboral, Synasterobe, Synasteron, and Zenalosyn.[17][18][9][3][23]
Availability
United States
Oxymetholone is one of the few AAS that remains available for medical use in the United States.[24] The others (as of November 2017) are testosterone, testosterone cypionate, testosterone enanthate, testosterone undecanoate, methyltestosterone, fluoxymesterone, and oxandrolone.[24]
Other countries
The availability of oxymetholone is fairly limited and seems to be scattered into isolated markets in Europe, Asia, and North and South America.[3] It is known to be available in Turkey, Greece, Moldova, Iran, Thailand, Brazil, and Paraguay.[3][9] At least historically, it has also been available in Canada, the United Kingdom, Belgium, the Netherlands, Spain, Poland, Israel, Hong Kong, and India.[18]
Legal status
Oxymetholone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[25]
References
- Saartok T, Dahlberg E, Gustafsson JA (1984). "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology. 114 (6): 2100–6. doi:10.1210/endo-114-6-2100. PMID 6539197.
- Maryanne Hochadel; Mosby (1 April 2015). Mosby's Drug Reference for Health Professions. Elsevier Health Sciences. pp. 1221–. ISBN 978-0-323-31103-8.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 323–334. ISBN 978-0-9828280-1-4.
- Pavlatos AM, Fultz O, Monberg MJ, Vootkur A, Pharmd (2001). "Review of oxymetholone: a 17alpha-alkylated anabolic-androgenic steroid". Clin Ther. 23 (6): 789–801, discussion 771. doi:10.1016/s0149-2918(01)80070-9. PMID 11440282.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Zderic, John A.; Carpio, Humberto; Ringold, H. J. (January 1959). "Steroids. CVI. Synthesis of 7β-Methyl Hormone Analogs". Journal of the American Chemical Society. 81 (2): 432–436. doi:10.1021/ja01511a041.
- "Advertisements" (PDF). Proceedings of the Royal Society of Medicine. 54 (3). 1961. PMC 1870224.
- "Advertisements" (PDF). British Medical Journal. 1 (5224). 1961. PMC 1953122.
- https://www.drugs.com/international/Oxymetholone.html
- http://adisinsight.springer.com/drugs/800010476
- "Anadrol-50" (PDF). Meda Pharmaceuticals. December 2006. Retrieved 8 January 2012.
- "Oxymetholone Side Effects". drugs.com.
- "Anadrol Official FDA Information, Side Effects and Uses". drugs.com.
- Hengge UR, Stocks K, Wiehler H, Faulkner S, Esser S, Lorenz C, Jentzen W, Hengge D, Goos M, Dudley RE, Ringham G (2003). "Double-blind, randomized, placebo-controlled phase III trial of oxymetholone for the treatment of HIV wasting". AIDS. 17 (5): 699–710. doi:10.1097/00002030-200303280-00008. PMID 12646793.
- CORTESGALLEGOS, V., CASTANEDA, G., ALONSO, R., PEREZPASTEN, E., REYESLUGO, V., BARRON, C., ... & VILLALPANDO, S. (1982, January). SPONTANEOUS AND OXYMETHOLONE-INDUCED GYNECOMASTIA. In JOURNAL OF ANDROLOGY (Vol. 3, No. 1, pp. 33-33). C/O ALLEN PRESS, INC PO BOX 368, LAWRENCE, KS 66044: AMER SOC ANDROLOGY, INC.
- VILLALPANDO, S., Mondragon, L., Barron, C., REYESLUGO, U., PEREZPASTEN, E., Alonso, R., ... & GALLEGOS, V. (1982, January). 5-ALPHA REDUCTASE BLOCKADE MAY BE RESPONSIBLE FOR SPONTANEOUS AND OXYMETHOLONE-INDUCED GYNECOMASTIA. In ARCHIVOS DE INVESTIGACION MEDICA (Vol. 13, No. 2, pp. S13-S13). SOCIAL APDO POSTAL 73-032, MEXICO DF 03020, MEXICO: INST MEXICANO SEGURO.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 924–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 779–. ISBN 978-3-88763-075-1.
- Locum, R (1961). "Latest Pharmaceutical Preparations" (PDF). The Central African Journal of Medicine. 7 (11): 443–444.
- CLARK GM (1962). "New drugs in rheumatic disease". Arthritis Rheum. 5 (4): 415–8. doi:10.1002/art.1780050411. PMID 13879693.
- Matusow, Paul D (1962). "If - Then; C.A.M.S.I.; In the future" (PDF). Dalhousie Medical Journal. 15 (1).
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 212–. ISBN 978-94-011-4439-1.
- Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 632–. ISBN 978-3-642-66353-6.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 17 December 2016.
- Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.CS1 maint: multiple names: authors list (link)
Further reading
- Pavlatos AM, Fultz O, Monberg MJ, Vootkur A, Pharmd (2001). "Review of oxymetholone: a 17alpha-alkylated anabolic-androgenic steroid". Clin Ther. 23 (6): 789–801, discussion 771. doi:10.1016/s0149-2918(01)80070-9. PMID 11440282.