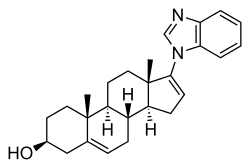
Galeterone
Galeterone (developmental code names TOK-001, VN/124-1) is a steroidal antiandrogen which was under development by Tokai Pharmaceuticals for the treatment of prostate cancer.[1] It possesses a unique dual mechanism of action, acting as both an androgen receptor antagonist and a CYP17A1 inhibitor, the latter of which prevents the biosynthesis of androgens.[2] As a CYP17A1 inhibitor, galeterone shows selectivity for 17,20-lyase over 17α-hydroxylase.[3]
 | |
| Clinical data | |
|---|---|
| Other names | TOK-001; VN/124-1; 17-(1H-Benzimidazol-1-yl)androsta-5,16-dien-3β-ol |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.233.599 |
| Chemical and physical data | |
| Formula | C26H32N2O |
| Molar mass | 388.555 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Galeterone was being compared to enzalutamide in a phase III clinical trial (ARMOR3-SV) for AR-V7-expressing metastatic castration-resistant prostate cancer.[4][5] Tokai announced the discontinuation of ARMOR3-SV on July 26, 2016, after a data monitoring committee determined that the trial was unlikely to meet its endpoint.[6] On August 22, 2016, the company announced the discontinuation of their phase II expansion (ARMOR2) as well.[7]
In the week following cancellation of the ARMOR3-SV clinical trial, Tokai announced a reduction of its workforce by around 60% to a total of 10 "full-time equivalent employees."[8] On December 22, 2016, a definitive share purchase agreement was announced, under which shareholders of Otic Pharma Ltd. would become the majority owners of Tokai Pharmaceuticals Inc., resulting in a NASDAQ-listed company (OticPharma, Inc.) focused on the development and commercialization of products for ear, nose, and throat disorders.[9]
In August 2017, Tokai Pharmaceuticals discontinued the development of galeterone.[1] On December 17 2018, it was announced that Educational & Scientific, LLC (ESL), in conjunction with University of Maryland ventures, would develop the drug.[10][11][12]
Prostate cancer drug abiraterone and its analog Galaterone are Δ5,3β-hydroxy steroids. Structures of these two agents are identical to endogenous steroid substrates (cholesterole, dehydroepiandorosterone and pregnenolone) for the 3β-hydroxysteroid dehydrogenase (3βHSD) of endocrine system. It has been reported that both of these agents are metabolized to 3-oxo-Δ4-steroids by 3βHSD in short period on oral administration. First metabolite 3-oxo-Δ4-abiraterone has more potent anti-prostate cancer properties than abiraterone where galeterone metabolite (3-oxo-Δ4-galaterone) has activity comparable to parent. Further these two metabolites undergo metabolism by 5α-reductase (5α-SRD) of endocrine system which leads to five more biologically inactive metabolites (Ref: Cell Chem. Biol. 2017, 24(7), 825; DOI: 10.1016/j.chembiol.2017.05.020). It is known that galeterone has poor oral bioavailability in rodents. Poor pharmacokinetic properties (oral absorption and metabolic half-life) of galeterone may be the reason for its clinical compromise.
Galeterone, along with abiraterone acetate, has been identified as an inhibitor of sulfotransferases (SULT2A1, SULT2B1b, SULT1E1), which are involved in the sulfation of dehydroepiandrosterone and other steroids and compounds, with Ki values in the sub-micromolar range.[13]
References
- http://adisinsight.springer.com/drugs/800031243
- Brawer MK (2008). "New treatments for castration-resistant prostate cancer: highlights from the 44th annual meeting of the American Society of Clinical Oncology, May 30–June 3, 2008, Chicago, IL". Rev Urol. 10 (4): 294–6. PMC 2615106. PMID 19145273.
- Yin L, Hu Q (2014). "CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents". Nat Rev Urol. 11 (1): 32–42. doi:10.1038/nrurol.2013.274. PMID 24276076.
- "A Study of Galeterone Compared to Enzalutamide In Men Expressing Androgen Receptor Splice Variant-7 mRNA (AR-V7) Metastatic CRPC - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-02-27.
- Silberstein, John L.; Taylor, Maritza N.; Antonarakis, Emmanuel S. (2016-04-01). "Novel Insights into Molecular Indicators of Response and Resistance to Modern Androgen-Axis Therapies in Prostate Cancer". Current Urology Reports. 17 (4): 29. doi:10.1007/s11934-016-0584-4. ISSN 1534-6285. PMC 4888068. PMID 26902623.
- http://www.businesswire.com/news/home/20160726005553/en/Tokai-Pharmaceuticals-Announces-Clinical-Update
- http://seekingalpha.com/news/3204773-tokai-pharma-flux-lead-product-candidate-galeterone-enrollment-mid-stage-prostate-cancer
- http://www.fiercebiotech.com/biotech/tokai-pharmaceuticals-takes-ax-to-60-workforce-after-phiii-blowout
- https://www.streetinsider.com/Corporate+News/Otic+Pharma+to+Become+Majority+Owner+of+Tokai+Pharma+(TKAI)/12366309.html
- "Lowe inks new deal for clinical trials of another cancer drug". Stock Gitter.
- "EDUCATIONAL & SCIENTIFIC, LLC AND UNIVERSITY OF MARYLAND, BALTIMORE EXPAND RELATIONSHIP THROUGH LICENSING AGREEMENT TO DEVELOP NOVEL PROSTATE CANCER TREATMENT". BioSpace. Retrieved 2019-04-03.
- "Jamaica Observer: Lowe and Team Granted Licence for Further Development of Prostate Cancer Drug". www.umventures.org. Retrieved 2019-04-03.
- Yip CK, Bansal S, Wong SY, Lau AJ (February 2018). "Identification of Galeterone and Abiraterone as Inhibitors of Dehydroepiandrosterone Sulfonation Catalyzed by Human Hepatic Cytosol, SULT2A1, SULT2B1b, and SULT1E1". Drug Metab. Dispos. doi:10.1124/dmd.117.078980. PMID 29436390.