Ligandrol
Ligandrol (VK5211, LGD-4033)[2] is an investigational selective androgen receptor modulator (SARM) for treatment of conditions such as muscle wasting and osteoporosis,[3] discovered by Ligand Pharmaceuticals and currently under development by Viking Therapeutics.[4][5]
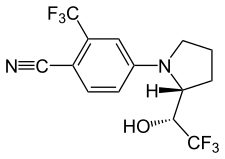 | |
| Clinical data | |
|---|---|
| Other names | VK5211; LGD-4033 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 24-36 hours [1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H12F6N2O |
| Molar mass | 338.253 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ligandrol has been found in World Anti-Doping Agency (WADA) samples and in racehorses too. Like anabolic steroids, it can stimulate muscle growth. "SARMs have shown superior side effect profiles compared with anabolic steroids, which arguably makes them attractive for use by individuals seeking an unfair advantage over their competitors".[6]
WADA control
At least since June 2015, ligandrol has been available via the internet. In that month, German scientists proposed a new test to detect its metabolites present in human urine, and suggested an expansion of the WADA regime.[7]
Misbranded as dietary supplement
On 23 October 2017, a nutritional supplement company in Missouri called Infantry Labs was warned by the FDA that the distribution of two of its products violated the Federal Food, Drug, and Cosmetic Act. One of the substances was ligandrol. The company advertised as benefits of the ligandrol: "increases in lean body mass and decrease in body fat" and "increases in strength, well being, as well as healing possibilities". The company mislabeled as "dietary supplements" what should have been "new drugs" or "prescription drugs" and were instructed to document the steps they would take in order to cease the violation.[8]
Also on 23 October 2017, the FDA sent a warning letter to a New Jersey company called Panther Sports Nutrition. The behaviour of the company was similar to the Infantry Labs case. The product was advertised as a "mass builder" and "physique enhancing agent".[9]
Clinical Research
According to a clinical trial conducted at the Boston Medical Center's Section of Endocrinology, Ligandrol can help improve lean body mass and muscle strength.[10]
Another study conducted in January 2013 found that Ligandrol can help increase lean muscle mass, and was generally well tolerated by test subjects.[10]
Adverse health effects
The FDA claims that "liver toxicity, adverse effects on blood lipid levels, and a potential to increase the risk of heart attack and stroke" are among the adverse health effects of ligandrol.[8]
Illicit use
Though not an approved drug, ligandrol has been sold on the black market.[11][12]
Ligandrol is currently on the World Anti-Doping Association list of prohibited drugs[13] and has been found in drug testing samples of some athletes.[14]
In 2015, the quarterback of the Florida Gators, Will Grier, was suspended for testing positive for ligandrol, a claim that the University of Florida denies.[15]
In 2017, Joakim Noah was banned for twenty games by the NBA for testing positive for ligandrol.[16]
In 2019, Australian swimmer Shayna Jack tested positive for ligandrol. She denies knowingly taking the substance.[17]
In August 2019, it came to light that Canadian sprint canoeist Laurence Vincent Lapointe tested positive for ligandrol; the athlete denies knowingly taking a forbidden substance that resulted in her suspension from competition. The athlete remarked that the National Team Training Centre purchased nutritional supplements for its athletes and denied buying or taking nutritional supplements on her own.[18]
Commercial environment
Viking Therapeutics does not currently manufacture any pharmaceutical products, but is developing several products to treat metabolic and endocrine disorders. Thus, the company relies upon investments from stockholders and business partners. The company completed its Initial Public Offering in the 2nd quarter of Fiscal Year 2015, generating $22.3M in net proceeds.[19] Viking Therapeutics assumed the largest expense in 2014 from cited Research and Development costs ($21.2M), which may be related to purchase of required equipment and facilities to develop the drug candidates licensed by Ligand Pharmaceuticals in that year, including ligandrol.[19]
Financial risks of development
Viking Therapeutics has yet to bring a drug to market. If one or several of their drug candidates does not pass clinical trials before one is successfully brought to market, then a significant amount of stockholders would likely sell their stocks, seriously reducing revenue and capability to continue development of ligandrol. Additionally, as noted in the Form 10-Q submitted on 10 November 2016, Viking currently relies heavily upon licensed technologies by Ligand Pharmaceutics. If Viking Therapeutics were to lose this license, then its operating capability would be severely impacted.[20]
Other commercial aspects
Ligand Pharmaceutics has disclosed that its royalty fee incurred upon Viking Therapeutics for production and sale of ligandrol would be 7.25-9.25% of the product revenue.[21]
Another promising drug candidate, VK2809, is expected to drive a major shift in the market cap of Viking Therapeutics and its share of the market for TRβ agonists, which is currently dominated by Madrigal Pharmaceuticals.[22] This would improve the company portfolio as a whole, and would allow for greater capitalization upon the potential success of ligandrol.
Intellectual property
Ligand Pharmaceuticals has patents filed for the manufacture and therapeutic use of certain compounds occupying several stated chemical structures, which are intended for use as selective androgen receptor modulators. The patent is filed under the following designations: US8519158 B2, US8865918, US9359285, US20070254875, US20140005186, US20150099720, and WO2005090282A1. The patents will expire on March 12, 2025. These patents effectively protect any future capitalization upon ligandrol in the market by Viking and Ligand through their licensing agreement.[23]
Regulatory information
In the United States, ligandrol is currently an Investigational New Drug.
Pre-clinical
Oral administration of the drug to cynomolgus monkeys at daily doses varying from 0 to 75 mg/kg over 13 weeks demonstrated significant body weight gain in both males and females. After 48 days, the 75 mg/kg dose testing was halted due to toxicity concerns, but this did not negatively impact development as the dose is significantly higher than those being utilized in the Phase 2 clinical trial.[24]
Clinical trials
In a Phase 1 clinical trial of 76 adult male humans in which the dose size was varied, a dose-dependent increase in lean body mass was observed with no significant adverse events over 21 days.[2]
The Phase 2 clinical trial, initiated on 3 November 2016, consists of 120 patients recovering from hip fracture surgery. The randomized study participants will receive either a placebo or varying dose sizes of LGD-4033 over a period of 12 weeks, with improved lean body mass as the primary endpoint. Other endpoints include satisfactory results in terms of quality of life, safety, and pharmacokinetics.[25]
References
- Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, et al. (Jan 2013). "The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men". J Gerontol A Biol Sci Med Sci. 68 (1): 87–95. doi:10.1093/gerona/gls078. PMC 4111291. PMID 22459616.
- Basaria, S., L. Collins, E. L. Dillon, K. Orwoll, T. W. Storer, R. Miciek, J. Ulloor, A. Zhang, R. Eder, H. Zientek, G. Gordon, S. Kazmi, M. Sheffield-Moore, and S. Bhasin. "The Safety, Pharmacokinetics, and Effects of LGD-4033, a Novel Nonsteroidal Oral, Selective Androgen Receptor Modulator, in Healthy Young Men." The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 68.1 (2012): 87-95. Web. 21 Nov. 2016.
- . doi:10.1093/gerona/gls078. Cite journal requires
|journal=(help); Missing or empty|title=(help) - "Ligand Presents New Preclinical Data on its Lead SARM Molecule LGD-4033 at the Gerontological Society of America Annual Meeting" (Press release). San Diego: Ligand Pharmaceuticals. November 20, 2009. Retrieved November 23, 2014.
- Viking Signs Broad Licensing Deal With Ligand Pharmaceuticals for Rights to Five Novel Therapeutic Programs
- . PMID 29334634. Cite journal requires
|journal=(help); Missing or empty|title=(help) - . doi:10.1002/rcm.7189. Cite journal requires
|journal=(help); Missing or empty|title=(help) - "WARNING LETTER Infantry Labs LLC MARCS-CMS 535333 — OCT 23, 2017". FDA. 23 October 2017.
- "WARNING LETTER Panther Sports Nutrition MARCS-CMS 535341 — OCT 23, 2017". FDA. 23 October 2017.
- Basaria, Shehzad; Collins, Lauren; Dillon, E. Lichar; Orwoll, Katie; Storer, Thomas W.; Miciek, Renee; Ulloor, Jagadish; Zhang, Anqi; Eder, Richard (January 2013). "The Safety, Pharmacokinetics, and Effects of LGD-4033, a Novel Nonsteroidal Oral, Selective Androgen Receptor Modulator, in Healthy Young Men". The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 68 (1): 87–95. doi:10.1093/gerona/gls078. ISSN 1079-5006. PMC 4111291. PMID 22459616.
- Geldof, Lore; Pozo, Oscar J.; Lootens, Leen; Morthier, Wouter; Van Eenoo, Peter; Deventer, Koen (2017). "In vitro metabolism study of a black market product containing SARM LGD-4033". Drug Testing and Analysis. 9 (2): 168–178. doi:10.1002/dta.1930. PMID 26767942.
- Krug, Oliver; Thomas, Andreas; Walpurgis, Katja; Piper, Thomas; Sigmund, Gerd; Schänzer, Wilhelm; ,aussmann, Tim; Thevis, Mario (2014). "Identification of black market products and potential doping agents in Germany 2010–2013". European Journal of Clinical Pharmacology. 70 (11): 1303–1311. doi:10.1007/s00228-014-1743-5. PMID 25168622.
- "Prohibited List".
- Cox, Holly D.; Eichner, Daniel (2017). "Detection of LGD-4033 and its metabolites in athlete urine samples". Drug Testing and Analysis. 9 (1): 127–134. doi:10.1002/dta.1986. PMID 27168428.
- Trahan, Kevin (12 October 2015). "Florida starting QB Will Grier suspended for at least 2015 after taking banned substance". SB Nation. Retrieved 20 October 2015.
- "NBA bans Joakim Noah 20 games for drug violation". Fox Sports. March 25, 2017.
- James Maasdorp (July 28, 2019). "Shayna Jack reveals banned substance Ligandrol was behind her doping suspension from swimming". Australian Broadcasting Corporation. Retrieved July 28, 2019.
- "Canada's Vincent Lapointe reveals she tested positive for muscle-building substance". CBC. 20 August 2019.
- Lian, Brian, Ph.D. "Q2 2015." Viking Therapeutics InvestorRoom. Viking Therapeutics, Inc., 3 Aug. 2015. Web. 21 Nov. 2016.
- Lian, Brian, Ph.D. “Form 10-Q: Viking Therapeutics, Inc. –VKTX.” www.vikingtherapeutics.com (2016).Web. 21 November 2016.
- "Royalty Table :: Ligand Pharmaceuticals, Inc. (LGND)." Royalty Table :: Ligand Pharmaceuticals, Inc. (LGND). Ligand Pharmaceuticals, Inc., 21 Nov. 2016. Web. 21 Nov. 2016.
- Bautz, David, PhD. (16 May 2016). "VKTX: Phase 2 Study of VK5211 for Hip Fracture Ongoing; Phase 2 Study of VK2809 for Hypercholersterolemia to Commence in Mid-2016". Yahoo Finance.CS1 maint: uses authors parameter (link)
- Zhi, Lin, Robert I. Higuchi, Cornelis Arjan Van Oeveren, Thomas Lot Stevens Lau, and Ligand Pharmaceuticals Incorporated. Androgen Receptor Modulator Compounds and Methods. Ligand Pharmaceuticals Incorporated, assignee. Patent US 8519158 B2. 11 Mar. 2005. Print.
- Bautz, David, PhD. "VKTX: Additional Preclinical Data Shows Robust and Durable Weight Gain for VK5211-Treated Primates." Yahoo. Yahoo, Inc., 8 Dec. 2015. Web. 21 Nov. 2016.
- "Viking Therapeutics Initiates Phase 2 Trial of VK5211 in Patients Recovering From Hip Fracture." FierceBiotech. Questex LLC, 03 Nov. 2015. Web. 21 Nov. 2016.