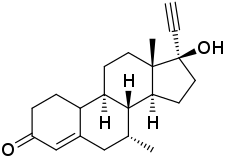
Δ4-Tibolone
Δ4-Tibolone (developmental code name ORG-OM-38), also known as 7α-methylnorethisterone or as 7α-methyl-17α-ethynyl-19-nortestosterone, is a synthetic androgen and progestin which was never marketed.[1][2] The compound is a major active metabolite of tibolone, which itself is a prodrug of δ4-tibolone along with 3α-hydroxytibolone and 3β-hydroxytibolone (which, in contrast to δ4-tibolone, are estrogens).[1] Tibolone and δ4-tibolone are thought to be responsible for the androgenic and progestogenic activity of tibolone, while 3α-hydroxytibolone and 3β-hydroxytibolone are thought to be responsible for its estrogenic activity.[1]
| Compound | Code name | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Noretynodrel | – | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Norethisterone (δ4-NYD) | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 3α-Hydroxynoretynodrel | – | ? | ? | ? | ? | ? | ? | ? |
| 3β-Hydroxynoretynodrel | – | ? | ? | ? | ? | ? | ? | ? |
| Ethinylestradiol | – | 15–25 | 1–3 | 112 | 1–3 | <1 | 0.18 | <0.1 |
| Tibolone (7α-Me-NYD) | ORG-OD-14 | 6 | 6 | 1 | ? | ? | ? | ? |
| Δ4-Tibolone | ORG-OM-38 | 90 | 35 | 1 | 0 | 2 | 1 | 0 |
| 3α-Hydroxytibolone | ORG-4094 | 0 | 3 | 4–6 | 0 | ? | ? | ? |
| 3β-Hydroxytibolone | ORG-301260 | 0 | 4 | 3–29 | 0 | ? | ? | ? |
| 7α-Methylethinylestradiol | – | ? | ? | ? | ? | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | ||||||||
| Compound | Code name | PR | AR | ERα | ERβ | GR | MR |
|---|---|---|---|---|---|---|---|
| Tibolone | ORG-OD-14 | 123 | 1.05 | 4 | 105 | 2410* | 170* |
| Δ4-Tibolone | ORG-OM-38 | 46 | 0.2 | 26 | 300 | 1760* | 30* |
| 3α-Hydroxytibolone | ORG-4094 | 2400* | 135* | 1.7 | 100 | ND* | 1160* |
| 3β-Hydroxytibolone | ORG-30126 | 20000* | ND* | 2.4 | 115 | ND* | 3310* |
| Notes: Values are affinities (nM). Non-italicized values are EC50. Italicized values with an asterisk (*) are IC50. Reference ligands (EC50) were promegestone (5 nM for PR), metribolone (0.1 nM for AR), estradiol (0.018 nM for ERα, 0.08 nM for ERβ), dexamethasone (6 nM for GR), and aldosterone (0.36 nM for MR). Sources: See template. | |||||||
 | |
 | |
| Clinical data | |
|---|---|
| Other names | ORG-OM-38; Delta-4-Tibolone; 7α-Methylnorethisterone; 7α-Methyl-17α-ethynyl-19-nortestosterone; 17α-Ethynyl-17β-hydroxy-7α-methyl-4-estren-3-one |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Escande A, Servant N, Rabenoelina F, Auzou G, Kloosterboer H, Cavaillès V, Balaguer P, Maudelonde T (2009). "Regulation of activities of steroid hormone receptors by tibolone and its primary metabolites". J. Steroid Biochem. Mol. Biol. 116 (1–2): 8–14. doi:10.1016/j.jsbmb.2009.03.008. PMID 19464167.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.