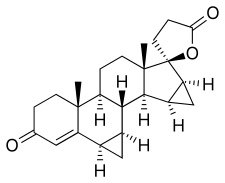
Drospirenone
Drospirenone, sold under the brand names Slynd among others, is a progestin medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses.[1][5] It available by itself and in combination with an estrogen.[5][6] The medication is taken by mouth.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alone: Slynd Combinations: Angeliq, Yasmin, Yasminelle, Yaz, others |
| Other names | Dihydrospirenone; Dihydrospirorenone; 1,2-Dihydrospirorenone; MSp; SH-470; ZK-30595; 17β-Hydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Antimineralocorticoid; Steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 76–85%[1][2] |
| Protein binding | 95–97% (to albumin, not to SHBG or CBG)[1][2] |
| Metabolism | Liver (mostly CYP450-independent (reduction, sulfation, and cleavage of lactone ring); minor CYP3A4 contribution (<10%))[2][3][4] |
| Elimination half-life | 25–33 hours[2] |
| Excretion | Urine, feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.599 |
| Chemical and physical data | |
| Formula | C24H30O3 |
| Molar mass | 366.493 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include acne, headache, breast tenderness, weight increase and menstrual changes.[6] Rare side effects include high potassium levels and blood clots, among others. Drospirenone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has additional antimineralocorticoid and antiandrogenic activity and no other important hormonal activity.[1] Because of its antimineralocorticoid activity, drospirenone is said to more closely resemble bioidentical progesterone than other progestins.[7][8]
Drospirenone was patented in 1976 and introduced for medical use in 2000.[9][10] It is available widely throughout the world.[5] The medication is sometimes referred to as a "fourth-generation" progestin.[11][12] It is available as a generic medication.[13] In 2016 the version with ethinylestradiol was the 109th most prescribed medication in the United States with more than 6 million prescriptions.[14]
Medical uses
Drospirenone is an ingredient in some birth control pills and is used in menopausal hormone therapy. In combination with ethinylestradiol it is used as contraception, and for women who want contraception it is also approved by the U.S. Food and Drug Administration (FDA) to treat moderate acne and premenstrual dysphoric disorder.[15] Although FDA-approved, this does come with a higher risk of blood clots than with other contraceptives containing other progestins, and therefore all individuals would have to be individually assessed for appropriateness. Studies have found that the Yaz formulation was superior to placebo in reducing premenstrual emotional and physical symptoms while also improving quality of life.[16]
Angeliq, a drospirenone and estradiol combination, has also been approved by the Food and Drug Administration (FDA) for treatment of moderate to severe vasomotor symptoms and/or vaginal atrophy associated with menopause.[17]
The FDA has several approved indications for combined estrogen and drospirenone preparations. They are approved as a first-line therapy for menopausal symptoms such as the relief of hot flashes.[18] Additionally, they have been shown to reduce the occurrence of bone fractures in postmenopausal women.[19]
Available forms
Drospirenone is sold as a combined birth control pill under the brand names Yasmin (US, EU, Latin America), Jasmine (France), Yarina (Russia)[20] in a dosage containing drospirenone 3 mg/ethinylestradiol 30 μg. In the United States, Bayer Schering released a pill based on Yasmin with the B vitamin folate (B9), which is marketed under the names Safyral and Beyaz. Worldwide it is also sold under the brand names Yaz and Yasminelle in a lower dosage containing drospirenone 3 mg/ethinylestradiol 20 μg. The drug is also available in combination with estradiol for use in menopausal hormone therapy. Drospirenone is not available on its own (i.e., as a standalone medication).[5]
Formulations
Drospirenone is a component of oral contraceptive formulations including the following:
- Yasmin/Jamine/Yarina contains 3 mg drospirenone and 30 μg ethinylestradiol per tablet. It is indicated for the prevention of pregnancy in women who elect an oral contraceptive.
- Safyral contains 3 mg drospirenone and 30 μg ethinylestradiol per tablet. It is indicated for the prevention of pregnancy in women who elect an oral contraceptive as well as to provide a daily dose of folate supplementation, which is recommended for women in their reproductive years. Folate lowers the risk of having rare neural tube birth defects in a pregnancy occurring during Safyral use or shortly after stopping.[21]
- Yaz/Gianvi/Vestura contains 3 mg drospirenone and 20 μg ethinylestradiol per tablet. It is indicated for prevention of pregnancy as well as treatment of premenstrual dysphoric disorder for women who choose to use an oral contraceptive for contraception. There has also been evidence for this formulation to treat moderate acne for women 14 years of age or older who choose to use an oral contraceptive for birth control.[22]
- A complete list of FDA approved oral contraceptives containing drospirenone as of October 2012: Beyaz (Drospirenone 3 mg, ethinylestradiol 0.02 mg and levomefolate calcium 0.451 mg), Drospirenone and ethinylestradiol (Drospirenone 3 mg and ethinylestradiol 0.03 mg), Gianvi (Drospirenone 3 mg and ethinylestradiol 0.02 mg), Loryna (Drospirenone 3 mg and ethinylestradiol 0.02 mg), Ocella (Drospirenone 3 mg and ethinylestradiol 0.03 mg), Safyral (Drospirenone 3 mg, ethinylestradiol 0.03 mg, and levomefolate calcium 0.451 mg), Syeda (Drospirenone 3 mg and ethinylestradiol 0.03 mg), Yasmin (Drospirenone 3 mg and ethinylestradiol 0.03 mg), Zarah (Drospirenone 3 mg and ethinylestradiol 0.03 mg), Yaz (Drospirenone 3 mg and ethinylestradiol 0.02 mg)[23]
Contraindications
In addition to contraindications common to all combined estrogen–progestin medications, drospirenone-containing medications are contraindicated in women with severe chronic kidney disease according to European Medicines Agency (EMA)-approved labels,[24] and contraindicated in women with chronic kidney disease, adrenal insufficiency, or liver disease according to FDA-approved labels.[25]
Side effects
High potassium levels
Drospirenone is an antimineralocorticoid with potassium-sparing properties, though in most cases no increase of potassium levels is to be expected.[24] In women with mild or moderate chronic kidney disease, or in combination with chronic daily use of other potassium-sparing medications (ACE inhibitors, angiotensin II receptor antagonists, potassium-sparing diuretics, heparin, antimineralocorticoids, or nonsteroidal anti-inflammatory drugs), a potassium level should be checked after two weeks of use to test for hyperkalemia.[24][26]
Blood clots
While all oral contraceptives can increase the risk for venous thromboembolic events, including fatal blood clots, several studies have reported a greater risk for women taking contraceptives containing drospirenone. Women who take contraceptive pills containing drospirenone have a 6- to 7-fold risk of developing thromboembolism (dangerous blood clots) compared to women who do not take any contraceptive pill, and have twice the risk (some epidemiological studies suggest thrice, according to the FDA) compared to women who take a contraceptive pill containing levonorgestrel. However, the absolute risk is small, in the neighborhood of 9 to 27 out of 10,000 women on an oral contraceptive for a year (up to 9 for levonorgestrel vs. up to 27 for drospirenone, or about 0.09% vs 0.3% per year).[27][28]
When the U.S. Food and Drug Administration (FDA) became concerned about the risks of drospirenone, they funded studies based on the medical records of more than 800,000 women taking oral contraceptives. They found that the risk of VTE, which includes dangerous and potentially fatal blood clots, was 93% higher for women who had been taking oral contraceptives containing drospirenone for only 3 months or less and 290% higher for women taking drospirenone-containing oral contraceptives for 7 to 12 months, compared to women taking other types of oral contraceptives.[29]
The FDA recently updated the label for contraceptives containing drospirenone to include warnings for stopping use prior to and after surgery, and to warn that contraceptives with drospirenone may have a higher risk of dangerous blood clots.[25]
Overdose
Interactions
Pharmacology
Pharmacodynamics
Drospirenone binds with high affinity to the progesterone receptor (PR) and mineralocorticoid receptor (MR), with lower affinity to the androgen receptor (AR), and with very low affinity to the glucocorticoid receptor (GR).[1][30][31] It is an agonist of the PR and an antagonist of the MR and AR, and hence, is a progestogen, antimineralocorticoid, and antiandrogen.[1][30] Drospirenone has no estrogenic activity and no appreciable glucocorticoid or antiglucocorticoid activity.[1][30] Progestogenic, antimineralocorticoid, and mild antiandrogenic effects have been observed in humans with drospirenone at a dosage of 0.5 to 4 mg/day.[32]
Progestogenic activity
Drospirenone is an agonist of the PR, the biological target of progestogens like progesterone.[1][30] It has about 35% of the affinity of promegestone for the PR and about 70% of the affinity of progesterone for the PR.[1] Drospirenone has antigonadotropic and functional antiestrogenic effects as a result of PR activation.[1][30] The ovulation-inhibiting dosage of drospirenone is 2.0 mg/day.[1] The medication acts as a contraceptive by activating the PR, which suppresses the secretion of luteinizing hormone, inhibits ovulation, and alters the cervical membrane and endometrium.[33]
Antimineralocorticoid activity
Drospirenone is an antagonist of the MR, the biological target of mineralocorticoids like aldosterone, and hence is an antimineralocorticoid.[30] It has about 230% of the affinity of aldosterone and progesterone for the MR.[1] Drospirenone is 8 to 10 times more potent as an antimineralocorticoid than spironolactone.[30][32] As such, 3 mg drospirenone is equivalent to about 20 to 25 mg spironolactone in terms of antimineralocorticoid activity.[34] The antimineralocorticoid activity exhibited by drospirenone promotes sodium excretion and decreases fluid retention.[35] It has been said that the pharmacological profile of drospirenone more closely resembles that of progesterone than other progestins, because its antimineralocorticoid activity is a property that it uniquely shares with progesterone.[30]
Antiandrogenic activity
Drospirorenone is an antagonist of the AR, the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1] It has about 65% of the affinity of the synthetic anabolic steroid metribolone for the AR.[1] The medication is more potent as an antiandrogen than spironolactone, but is less potent than cyproterone acetate, with about 30% of its antiandrogenic activity.[1][30][32]
Pharmacokinetics
The oral bioavailability of drospirenone is between 76 and 85%.[1] Peak levels occur 1 to 2 hours after an oral dose.[1] There is some accumulation in drospirenone levels with continuous administration, with steady-state levels of drospirenone achieved after 7 to 10 days of combined ethinylestradiol and drospirenone therapy.[1] The plasma protein binding of drospirenone is 95 to 97%, with a majority bound to albumin and 3 to 5% circulating freely or unbound.[1] It has no affinity for sex hormone-binding globulin or corticosteroid-binding globulin, and hence is not bound by these plasma proteins.[1] Drospirenone is metabolized by opening of its lactone ring to form an acid grou and by reduction of its double bond between the C4 and C5 positions.[1] The biological half-life of drospirenone is between 25 and 33 hours.[2]
Chemistry
Chemical structures of spirolactones
|
Drospirenone, also known as 1,2-dihydrospirorenone or as 17β-hydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone, is a synthetic steroidal 17α-spirolactone, or more simply a spirolactone.[5][36] It is an analogue of other spirolactones like spironolactone, canrenone, and spirorenone.[5][36] Drospirenone differs structurally from spironolactone only in that the C7α acetylthio substitution of spironolactone has been removed and two methylene groups have been substituted in at the 6β,7β and 15β,16β positions.[37]
The loss of the C7α acetylthio group of spironolactone, a compound with negligible progestogenic activity,[38][39] appears to be involved in the restoration of progestogenic activity in drospirenone, as SC-5233, the analogue of spironolactone without a C7α substitution, has potent progestogenic activity similarly to drospirenone.[40]
History
Drospirenone was introduced for medical use in 2000.[9] It is sometimes described as a "fourth-generation" progestin based on its time of introduction.[11][12]
Society and culture
Generic names
Drospirenone is the generic name of the drug and its INN, USAN, BAN, and JAN, while drospirénone is its DCF.[5] Its name is a shortened form of the name 1,2-dihydrospirorenone or dihydrospirenone.[5][36] Drospirenone is also known by its developmental code names SH-470 and ZK-30595 (alone), BAY 86-5300, BAY 98-7071, and SH-T-00186D (in combination with ethinylestradiol), and BAY 86-4891 (in combination with estradiol).[5][36][41][42][43][44]
Brand names
Drospirenone is marketed in combination with an estrogen under a variety of brand names throughout the world.[5] Among others, it is marketed in combination with ethinylestradiol under the brand names Yasmin and Yaz and in combination with estradiol under the brand name Angeliq.[5]
Availability
Drospirenone is marketed widely throughout the world.[5]
Litigation
In July 2012, Bayer notified its stockholders that there were more than 12,000 lawsuits against the company involving Yaz, Yasmin, and other oral contraceptives with drospirenone, and that the company by then settled 1,977 cases for US$402.6 million, for an average of US$212,000 per case, while setting aside US$610.5 million to settle the others.[45]
As of July 17, 2015, there have been at least 4,000 lawsuits and claims still pending regarding venous thromboembolic events. This doesn't include the roughly 10,000 claims that Bayer has already settled without admitting liability. These claims of venous thromboembolic events have amounted to US$1.97 billion. Bayer also reached a settlement for arterial thromboembolic events, including stroke and heart attacks, for US$56.9 million. [46]
Research
Drospirenone (developmental code name LF-111) is or was under development by Leon Farma as a progestin-only pill for hormonal birth control in women, but as of March 2017 no recent reports of development have been identified.[47] The formulation has reached phase III clinical trials for this indication.[47]
Drospirenone (tentative brand name Estelle) is under development by Mithra Pharmaceuticals in combination with estetrol as a combined oral contraceptive for pregnancy in women.[48] As of July 2018, it is in phase III clinical trials.[48]
References
- Kuhl, H (2009). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (sup1): 3–63. doi:10.1080/13697130500148875. ISSN 1369-7137. PMID 16112947.
- Krattenmacher, Rolf (2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. ISSN 0010-7824. PMID 11024226.
- Bachmann, Gloria (2009). "Drospirenone/ethinyl estradiol 3 mg/20 µg (24/4 day regimen): hormonal contraceptive choices – use of a fourth-generation progestin". Patient Preference and Adherence. 3: 259–64. doi:10.2147/PPA.S3901. ISSN 1177-889X. PMC 2778416. PMID 19936169.
- Wiesinger, Herbert; Berse, Matthias; Klein, Stefan; Gschwend, Simone; Höchel, Joachim; Zollmann, Frank S.; Schütt, Barbara (2015). "Pharmacokinetic interaction between the CYP3A4 inhibitor ketoconazole and the hormone drospirenone in combination with ethinylestradiol or estradiol". British Journal of Clinical Pharmacology. 80 (6): 1399–1410. doi:10.1111/bcp.12745. ISSN 0306-5251. PMC 4693482. PMID 26271371.
- "Drospirenone".
- "Drospirenone tablets, for oral use" (PDF). FDA. Retrieved 12 November 2019.
- Oelkers W (December 2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". Eur J Contracept Reprod Health Care. 5 Suppl 3: 17–24. PMID 11246598.
- Oelkers W (December 2002). "Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone". Eur J Contracept Reprod Health Care. 7 Suppl 3: 19–26, discussion 42–3. PMID 12659403.
- Enrique Ravina (11 January 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 193–. ISBN 978-3-527-32669-3.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 459. ISBN 9783527607495.
- Robert Anthony Hatcher; Anita L. Nelson, M.D. (2007). Contraceptive Technology. Ardent Media. pp. 196–. ISBN 978-1-59708-001-9.
- James Q. Del Rosso; Joshua A. Zeichner (20 April 2016). Advances in Acne Management, An Issue of Dermatologic Clinics, E-Book. Elsevier Health Sciences. pp. 160–. ISBN 978-0-323-41753-2.
- "Generic Yasmin Availability".
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- Cerner Multum, Inc. (June 11, 2012). "drospirenone and ethinyl estradiol". Auckland, New Zealand: Drugs.com. Retrieved October 24, 2011.
- Lanza di Scalea, Teresa (June 2017). "Premenstrual Dysphoric Disorder". Psychiatric Clinics of North America. 40 (2): 201–206. doi:10.1016/j.psc.2017.01.002. PMID 28477648.
- "ANGELIQ Tablets" (PDF). Food and Drug Administration.
- Maclennan, A. H.; Broadbent, J. L.; Lester, S.; Moore, V. (18 October 2004). "Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes". The Cochrane Database of Systematic Reviews (4): CD002978. doi:10.1002/14651858.CD002978.pub2. ISSN 1469-493X. PMID 15495039.
- Torgerson, D. J.; Bell-Syer, S. E. (13 June 2001). "Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials". JAMA. 285 (22): 2891–2897. doi:10.1001/jama.285.22.2891. ISSN 0098-7484. PMID 11401611.
- "Daylette Ethinylestradiolum, Drospirenonum - ulotka - dawkowanie, zastosowanie, opis leku - www.doz.pl".
- "Yasmin/Safyral". Retrieved February 9, 2015.
- "Yaz" (PDF). Retrieved October 31, 2017.
- Research, Center for Drug Evaluation and. "Drug Safety and Availability - FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone". www.fda.gov. Retrieved 2017-11-07.
- Bayer (March 25, 2013). "Summary of Product Characteristics (SPC): Yasmin". London: electronic Medicines Compendium (eMC), Datapharm. Retrieved April 24, 2014.
4.3. Contraindications: • Severe chronic kidney disease or acute kidney failure. • Presence or history of severe hepatic disease as long as liver function values have not returned to normal.
- Bayer (April 10, 2012). "Yasmin full prescribing information" (PDF). Silver Spring, Md.: Food and Drug Administration (FDA). Retrieved April 14, 2012.
4. Contraindications: • Renal impairment. • Adrenal insufficiency. • Liver disease.
- Nelson, Anita L.; Cwiak, Carrie (2011). "Combined oral contraceptives (COCs)". In Hatcher, Robert A.; Trussell, James; Nelson, Anita L.; Cates, Willard Jr.; Kowal, Deborah; Policar, Michael S. (eds.). Contraceptive Technology (20th revised ed.). New York: Ardent Media. pp. 249–341. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734.
- Lidegaard, Øjvind; Milsom, Ian; Geirsson, Reynir Tomas; Skjeldestad, Finn Egil (July 2012). "Hormonal contraception and venous thromboembolism" (PDF). Acta Obstetricia et Gynecologica Scandinavica. 91 (7): 769–778. doi:10.1111/j.1600-0412.2012.01444.x. PMID 22568831. Retrieved October 22, 2012.
- Lidegaard, Øjvind; Milsom, Ian; Skovlund, Charlotte Wessel; Skjeldestad, Finn Egil; Løkkegaard, Ellen (October 25, 2011). "Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9" (PDF). BMJ. 343: d6423. doi:10.1136/bmj.d6423. PMC 3202015. PMID 22027398. Retrieved November 26, 2011.
- Dunn, Nick (April 21, 2011). "The risk of deep venous thrombosis with oral contraceptives containing drospirenone. Data are inconclusive, but alternatives may be preferable unless specifically contraindicated. (editorial)" (PDF). BMJ. 342: d2519. doi:10.1136/bmj.d2519. PMID 21511807. Retrieved April 25, 2011.
- Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E (1995). "Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity". Ann. N. Y. Acad. Sci. 761: 311–35. doi:10.1111/j.1749-6632.1995.tb31386.x. PMID 7625729.
- Fuhrmann, Ulrike; Krattenmacher, Rolf; Slater, Emily P.; Fritzemeier, Karl-Heinrich (1996). "The novel progestin drospirenone and its natural counterpart progesterone: Biochemical profile and antiandrogenic potential". Contraception. 54 (4): 243–251. doi:10.1016/S0010-7824(96)00195-3. ISSN 0010-7824. PMID 8922878.
- Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A (2003). "Conception and pharmacodynamic profile of drospirenone". Steroids. 68 (10–13): 891–905. doi:10.1016/j.steroids.2003.08.008. PMID 14667981.
- "Drospirenone". pubchem.ncbi.nlm.nih.gov.
- Hermann P.G. Schneider; Frederick Naftolin (22 September 2004). Climacteric Medicine - Where Do We Go?: Proceedings of the 4th Workshop of the International Menopause Society. CRC Press. pp. 133–. ISBN 978-0-203-02496-6.
- Genazzani, Andrea R.; Mannella, Paolo; Simoncini, Tommaso (February 2007). "Drospirenone and its antialdosterone properties". Climacteric. 10 (Supplement 1): 11–18. doi:10.1080/13697130601114891. PMID 17364593. Retrieved November 26, 2011.
- Martin Negwer; Hans-Georg Scharnow (4 October 2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 2539. ISBN 978-3-527-30247-5.
- Howard J.A. Carp (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. pp. 115–. ISBN 978-3-319-14385-9.
- Hu X, Li S, McMahon EG, Lala DS, Rudolph AE (2005). "Molecular mechanisms of mineralocorticoid receptor antagonism by eplerenone". Mini Rev Med Chem. 5 (8): 709–18. doi:10.2174/1389557054553811. PMID 16101407.
- Nakajima ST, Brumsted JR, Riddick DH, Gibson M (1989). "Absence of progestational activity of oral spironolactone". Fertil. Steril. 52 (1): 155–8. doi:10.1016/s0015-0282(16)60807-5. PMID 2744183.
- Hertz R, Tullner WW (1958). "Progestational activity of certain steroid-17-spirolactones". Proc. Soc. Exp. Biol. Med. 99 (2): 451–2. doi:10.3181/00379727-99-24380. PMID 13601900.
- "Ethinylestradiol/drospirenone - AdisInsight".
- "Ethinylestradiol/drospirenone/folic acid - AdisInsight".
- "Drospirenone/ethinylestradiol low-dose - Bayer HealthCare Pharmaceuticals - AdisInsight".
- "Estradiol/drospirenone - Bayer HealthCare Pharmaceuticals - AdisInsight".
- Feeley, Jef; Kresge, Naomi (July 31, 2012). "Bayer's Yasmin lawsuit settlements rise to $402.6 million". Bloomberg News. New York. Retrieved November 11, 2012.
- "Interim Report Second Quarter 2015".
- "Drospirenone - Leon Farma - AdisInsight".
- "Drospirenone/estetrol - Mithra Pharmaceuticals - AdisInsight".
Further reading
- Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- Oelkers W (December 2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". Eur J Contracept Reprod Health Care. 5 Suppl 3: 17–24. PMID 11246598.
- Oelkers W (February 2002). "The renin-aldosterone system and drospirenone". Gynecol. Endocrinol. 16 (1): 83–7. doi:10.1080/gye.16.1.83.87. PMID 11915587.
- Thorneycroft IH (November 2002). "Evolution of progestins. Focus on the novel progestin drospirenone". J Reprod Med. 47 (11 Suppl): 975–80. PMID 12497671.
- Dickerson V (November 2002). "Quality of life issues. Potential role for an oral contraceptive containing ethinyl estradiol and drospirenone". J Reprod Med. 47 (11 Suppl): 985–93. PMID 12497673.
- Oelkers W (December 2002). "Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone". Eur J Contracept Reprod Health Care. 7 Suppl 3: 19–26, discussion 42–3. PMID 12659403.
- Rübig A (October 2003). "Drospirenone: a new cardiovascular-active progestin with antialdosterone and antiandrogenic properties". Climacteric. 6 Suppl 3: 49–54. PMID 15018248.
- Oelkers W (March 2004). "Drospirenone, a progestogen with antimineralocorticoid properties: a short review". Mol. Cell. Endocrinol. 217 (1–2): 255–61. doi:10.1016/j.mce.2003.10.030. PMID 15134826.
- Heinemann LA, Dinger J (2004). "Safety of a new oral contraceptive containing drospirenone". Drug Saf. 27 (13): 1001–18. doi:10.2165/00002018-200427130-00003. PMID 15471507.
- Keam SJ, Wagstaff AJ (2003). "Ethinylestradiol/drospirenone: a review of its use as an oral contraceptive". Treat Endocrinol. 2 (1): 49–70. doi:10.2165/00024677-200302010-00005. PMID 15871554.
- Sitruk-Ware R (October 2005). "Pharmacology of different progestogens: the special case of drospirenone". Climacteric. 8 Suppl 3: 4–12. doi:10.1080/13697130500330382. PMID 16203650.
- Oelkers WH (October 2005). "Drospirenone in combination with estrogens: for contraception and hormone replacement therapy". Climacteric. 8 Suppl 3: 19–27. doi:10.1080/13697130500330341. PMID 16203652.
- Foidart JM (October 2005). "Added benefits of drospirenone for compliance". Climacteric. 8 Suppl 3: 28–34. doi:10.1080/13697130500330309. PMID 16203653.
- Christiansen C (October 2005). "Effects of drospirenone/estrogen combinations on bone metabolism". Climacteric. 8 Suppl 3: 35–41. doi:10.1080/13697130500330283. PMID 16203654.
- Whitehead M (March 2006). "Hormone replacement therapy with estradiol and drospirenone: an overview of the clinical data". J Br Menopause Soc. 12 Suppl 1: 4–7. doi:10.1258/136218006775992185. PMID 16513012.
- Shulman LP (June 2006). "A review of drospirenone for safety and tolerability and effects on endometrial safety and lipid parameters contrasted with medroxyprogesterone acetate, levonorgestrel, and micronized progesterone". J Womens Health (Larchmt). 15 (5): 584–90. doi:10.1089/jwh.2006.15.584. PMID 16796485.
- Palacios S, Foidart JM, Genazzani AR (November 2006). "Advances in hormone replacement therapy with drospirenone, a unique progestogen with aldosterone receptor antagonism" (PDF). Maturitas. 55 (4): 297–307. doi:10.1016/j.maturitas.2006.07.009. hdl:2268/9932. PMID 16949774.
- Archer DF (February 2007). "Drospirenone and estradiol: a new option for the postmenopausal woman". Climacteric. 10 Suppl 1: 3–10. doi:10.1080/13697130601114859. PMID 17364592.
- Genazzani AR, Mannella P, Simoncini T (February 2007). "Drospirenone and its antialdosterone properties". Climacteric. 10 Suppl 1: 11–8. doi:10.1080/13697130601114891. PMID 17364593.
- White WB (February 2007). "Drospirenone with 17beta-estradiol in the postmenopausal woman with hypertension". Climacteric. 10 Suppl 1: 25–31. doi:10.1080/13697130601114933. PMID 17364595.
- Motivala A, Pitt B (2007). "Drospirenone for oral contraception and hormone replacement therapy: are its cardiovascular risks and benefits the same as other progestogens?". Drugs. 67 (5): 647–55. doi:10.2165/00003495-200767050-00001. PMID 17385938.
- Archer DF (2007). "Drospirenone, a progestin with added value for hypertensive postmenopausal women". Menopause. 14 (3 Pt 1): 352–4. doi:10.1097/gme.0b013e31804d440b. PMID 17414576.
- Rapkin AJ, Winer SA (May 2007). "Drospirenone: a novel progestin". Expert Opin Pharmacother. 8 (7): 989–99. doi:10.1517/14656566.8.7.989. PMID 17472544.
- Archer DF (February 2007). "Drospirenone-containing hormone therapy for postmenopausal women. Perspective on current data". J Reprod Med. 52 (2 Suppl): 159–64. PMID 17477110.
- Mallareddy M, Hanes V, White WB (2007). "Drospirenone, a new progestogen, for postmenopausal women with hypertension". Drugs Aging. 24 (6): 453–66. doi:10.2165/00002512-200724060-00002. PMID 17571911.
- Fenton C, Wellington K, Moen MD, Robinson DM (2007). "Drospirenone/ethinylestradiol 3mg/20microg (24/4 day regimen): a review of its use in contraception, premenstrual dysphoric disorder and moderate acne vulgaris". Drugs. 67 (12): 1749–65. doi:10.2165/00003495-200767120-00007. PMID 17683173.
- Scheinfeld NS (2007). "Yaz (3 mg drospirenone/20 microg ethinyl estradiol)". Skinmed. 6 (6): 289. doi:10.1111/j.1540-9740.2007.07338.x. PMID 17975349.
- Foidart JM, Faustmann T (December 2007). "Advances in hormone replacement therapy: weight benefits of drospirenone, a 17alpha-spirolactone-derived progestogen". Gynecol. Endocrinol. 23 (12): 692–9. doi:10.1080/09513590701582323. PMID 18075844.
- Rapkin AJ, Sorger SN, Winer SA (February 2008). "Drospirenone/ethinyl estradiol". Drugs Today. 44 (2): 133–45. doi:10.1358/dot.2008.44.2.1191057. PMID 18389090.
- Pérez-López FR (June 2008). "Clinical experiences with drospirenone: from reproductive to postmenopausal years". Maturitas. 60 (2): 78–91. doi:10.1016/j.maturitas.2008.03.009. PMID 18468818.
- Bitzer J, Paoletti AM (2009). "Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: results of three multicentre trials". Clin Drug Investig. 29 (2): 73–8. doi:10.2165/0044011-200929020-00001. PMID 19133702.
- "Drospirenone in HRT?". Drug Ther Bull. 47 (4): 41–4. April 2009. doi:10.1136/dtb.2009.03.0011. PMID 19357298.
- Carranza-Lira S (2009). "Safety, efficacy and patient acceptability of drospirenone and estradiol in the treatment of menopausal vasomotor symptoms: a review". Clin Interv Aging. 4: 59–62. doi:10.2147/CIA.S4117. PMC 2685225. PMID 19503766.
- Simoncini T, Genazzani AR (February 2010). "A review of the cardiovascular and breast actions of drospirenone in preclinical studies". Climacteric. 13 (1): 22–33. doi:10.3109/13697130903437375. PMID 19938948.
- Sehovic N, Smith KP (May 2010). "Risk of venous thromboembolism with drospirenone in combined oral contraceptive products". Ann Pharmacother. 44 (5): 898–903. doi:10.1345/aph.1M649. PMID 20371756.
- Machado RB, Pompei Lde M, Giribela AG, Giribela CG (January 2011). "Drospirenone/ethinylestradiol: a review on efficacy and noncontraceptive benefits". Womens Health (Lond). 7 (1): 19–30. doi:10.2217/whe.10.84. PMID 21175386.
- Rapkin RB, Creinin MD (October 2011). "The combined oral contraceptive pill containing drospirenone and ethinyl estradiol plus levomefolate calcium". Expert Opin Pharmacother. 12 (15): 2403–10. doi:10.1517/14656566.2011.610791. PMID 21877996.
- Lopez LM, Kaptein AA, Helmerhorst FM (February 2012). "Oral contraceptives containing drospirenone for premenstrual syndrome". Cochrane Database Syst Rev (2): CD006586. doi:10.1002/14651858.CD006586.pub4. PMID 22336820.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ (June 2013). "Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review". BJOG. 120 (7): 801–10. doi:10.1111/1471-0528.12210. PMID 23530659.
- Toni I, Neubert A, Botzenhardt S, Gratzki N, Rascher W (September 2013). "Venous thromboembolism in adolescents associated with drospirenone-containing oral contraceptives - two case reports". Klin Padiatr. 225 (5): 266–7. doi:10.1055/s-0033-1353169. PMID 23975850.
- Han L, Jensen JT (October 2014). "Expert opinion on a flexible extended regimen of drospirenone/ethinyl estradiol contraceptive". Expert Opin Pharmacother. 15 (14): 2071–9. doi:10.1517/14656566.2014.949237. PMID 25186109.
- Idota N, Kobayashi M, Miyamori D, Kakiuchi Y, Ikegaya H (March 2015). "Drospirenone detected in postmortem blood of a young woman with pulmonary thromboembolism: A case report and review of the literature". Leg Med (Tokyo). 17 (2): 109–15. doi:10.1016/j.legalmed.2014.10.001. PMID 25454533.
- Lete I, Chabbert-Buffet N, Jamin C, Lello S, Lobo P, Nappi RE, Pintiaux A (2015). "Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: A literature review". Eur J Contracept Reprod Health Care. 20 (5): 329–43. doi:10.3109/13625187.2015.1050091. PMID 26007631.
- Zhao X, Zhang XF, Zhao Y, Lin X, Li NY, Paudel G, Wang QY, Zhang XW, Li XL, Yu J (September 2016). "Effect of combined drospirenone with estradiol for hypertensive postmenopausal women: a systemic review and meta-analysis". Gynecol. Endocrinol. 32 (9): 685–689. doi:10.1080/09513590.2016.1183629. PMID 27176003.
- Batur P, Casey PM (February 2017). "Drospirenone Litigation: Does the Punishment Fit the Crime?". J Womens Health (Larchmt). 26 (2): 99–102. doi:10.1089/jwh.2016.6092. PMID 27854556.
- Li J, Ren J, Sun W (March 2017). "A comparative systematic review of Yasmin (drospirenone pill) versus standard treatment options for symptoms of polycystic ovary syndrome". Eur. J. Obstet. Gynecol. Reprod. Biol. 210: 13–21. doi:10.1016/j.ejogrb.2016.11.013. PMID 27923166.
- Larivée N, Suissa S, Khosrow-Khavar F, Tagalakis V, Filion KB (September 2017). "Drospirenone-containing oral contraceptive pills and the risk of venous thromboembolism: a systematic review of observational studies". BJOG. 124 (10): 1490–1499. doi:10.1111/1471-0528.14623. PMID 28276140.
- Regidor PA, Schindler AE (October 2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.