Estradiol/progesterone
Estradiol/progesterone (E2/P4), sold under the brand names Bijuva and Juvenum, is a combined estrogen and progestogen medication which is used in the treatment of menopausal symptoms in postmenopausal women.[1] It contains estradiol, an estrogen, and progesterone, a progestogen, and is available in both oral and intramucular formulations.[1] E2/P4 differs from other estrogen–progestogen formulations in that the sex-hormonal agents used are bioidentical.
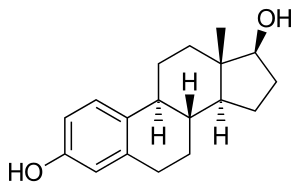 | |
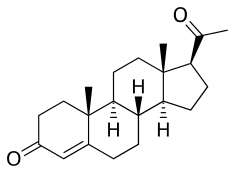 Estradiol (top) and progesterone (bottom) | |
| Combination of | |
|---|---|
| Estradiol | Estrogen |
| Progesterone | Progestogen |
| Clinical data | |
| Trade names | Bijuva, Juvenum |
| Other names | E2/P4; TX-001HR; TX-12-001HR; CA682-2 |
| Routes of administration | By mouth, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
Bijuva (formerly TX-001HR/TX-12-001HR) is an oral combination of estradiol (E2), an estrogen, and progesterone (P4), a progestogen, which was developed by TherapeuticsMD and is approved in the United States for the treatment of menopausal symptoms in women.[2][3] It is also under development for the treatment of endometrial hyperplasia in women.[2] The medication contains 2 mg solubilized E2 and 200 mg P4 in each gelatin capsule.[3][1] It is the first combination of E2 and P4 in oral capsule form that has been developed for clinical use.[3] Bijuva is currently in phase III clinical trials for endometrial hyperplasia.[2] The medication was approved by the Food and Drug Administration for the treatment of menopausal symptoms in October 2018.[2][4][1]
E2/P4 is available as an aqueous suspension of E2 and P4 encapsulated in microspheres for use by intramuscular injection once a month under the brand name Juvenum in Mexico.[5][6][7] It was introduced for the treatment and prevention of menopausal symptoms like hot flashes, vulvovaginal symptoms, and osteoporosis in December 2014.[5][6] The combination contains relatively low doses of E2 and P4 (1 mg and 20 mg, respectively) contained within microspheres that results in a slower release of the hormones.[5][6] Studies of this formulation have been published.[8][9]
E2/P4 with 5 mg E2 and 150 to 300 mg P4 encapsulated in microspheres in an aqueous suspension has been studied as a once-a-month combined injectable contraceptive but has not been further developed or introduced for medical use.[10][11][12][13][14][15] E2/P4 with 5 mg E2 and 100 mg P4 in a macrocrystalline aqueous suspension has also been studied as a once-a-month combined injectable contraceptive, but likewise was not further developed.[16][17]
See also
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210132s000lbl.pdf
- http://adisinsight.springer.com/drugs/800038089
- Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (2015). "Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy". Menopause. 22 (12): 1308–16. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- https://www.drugs.com/history/bijuva.html
- "Juvenum (estradiol/progesterone) - Medicamentos PLM". Medicamentos PLM. Archived from the original on 2018-09-17. Retrieved 2018-09-17.
- http://adisinsight.springer.com/drugs/800044558
- https://www.drugs.com/international/juvenum.html
- Cortés-Bonilla M, Alonso-Campero R, Bernardo-Escudero R, Francisco-Doce MT, Chavarín-González J, Pérez-Cuevas R, Chedraui P (October 2016). "Improvement of quality of life and menopausal symptoms in climacteric women treated with low-dose monthly parenteral formulations of non-polymeric microspheres of 17β-estradiol/progesterone". Gynecol. Endocrinol. 32 (10): 831–834. doi:10.1080/09513590.2016.1183628. PMID 27187320.
- Cortés-Bonilla M, Bernardo-Escudero R, Alonso-Campero R, Francisco-Doce MT, Hernández-Valencia M, Celis-González C, Márquez-Oñate R, Chedraui P, Uribe JA (July 2015). "Treatment of menopausal symptoms with three low-dose continuous sequential 17β-estradiol/progesterone parenteral monthly formulations using novel non-polymeric microsphere technology". Gynecol. Endocrinol. 31 (7): 552–9. doi:10.3109/09513590.2015.1019853. PMC 4776687. PMID 26062108.
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- Garza-Flores J, Fatinikun T, Hernandez L, Ramos I, Cardenas M, Menjivar M (July 1991). "A pilot study on the assessment of a progesterone/estradiol sustained release as once-a-month-injectable contraceptive". Contraception. 44 (1): 45–59. doi:10.1016/0010-7824(91)90105-O. PMID 1893701.
- Garza-Flores J, Hall PE, Perez-Palacios G (1991). "Long-acting hormonal contraceptives for women". J. Steroid Biochem. Mol. Biol. 40 (4–6): 697–704. doi:10.1016/0960-0760(91)90293-E. PMID 1958567.
- Alvarez-Sanchez, Francisco; Brache, Vivian; Faundes, Anibal (1993). "Recent experience with and future directions of contraceptive implants and injectable contraceptives". Current Opinion in Obstetrics and Gynecology. 5 (6): 805–814. doi:10.1097/00001703-199312000-00016. ISSN 1040-872X.
- Garza-flores, J.; Fatinikun, T.; Hernandez, L.; Ramos, I.; Cardenas, M.; Menjivar, M. (1991). "A pilot study on the assessment of a progesterone/estradiol sustained release as once-a-month-injectable contraceptive". Contraception. 44 (1): 45–59. doi:10.1016/0010-7824(91)90105-O. ISSN 0010-7824.
External links
- Bijuva (estradiol/progesterone) FDA label
- TX-001HR (Oral Estradiol + Progesterone) - TherapeuticsMD
- Estradiol/progesterone (Bijuva) - TherapeuticsMD - AdisInsight