Estradiol valerate/hydroxyprogesterone caproate
Estradiol valerate/hydroxyprogesterone caproate (EV/OHPC), sold under the brand names Gravibinon and Injectable No. 1 (or Chinese Injectable No. 1) among others, is a combined estrogen and progestogen medication which is used in the treatment of threatened miscarriage and other indications (e.g., as a means of pseudopregnancy)[1][2][3][3] and as a form of combined injectable birth control to prevent pregnancy.[4][5][6][7] It contains estradiol valerate (EV), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin.[8][4][5][6] The medication is given by injection into muscle once a day to once a month depending on the indication.[1][4][5][6]
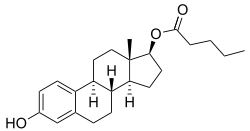 | |
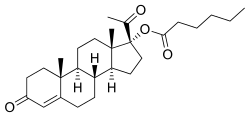 Estradiol valerate (top) and hydroxyprogesterone caproate (bottom) | |
| Combination of | |
|---|---|
| Estradiol valerate | Estrogen |
| Hydroxyprogesterone caproate | Progestogen |
| Clinical data | |
| Trade names | Gravibinon, Injectable No. 1, others |
| Other names | EV/OHPC; NSC-77622 |
| Routes of administration | Intramuscular injection |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
Medical uses
EV/OHPC has been used in the treatment of threatened miscarriage (habitual abortion) and for other indications under the brand name Gravibinon among others in Europe and Latin America.[1][2][9][3] The combination has also been used as a form of pseudopregnancy (high-dose estrogen/progestogen therapy), for instance to treat osteopenia due to hypogonadism, to induce feminization in hypogonadism/delayed puberty, and as a means of hormonal breast enhancement to produce breast enlargement.[10][11][12] EV/OHPC is administered daily to once per week or less often for the treatment of threatened miscarriage, for use as a form of pseudopregnancy, and for other indications.[10][11][12]
EV/OHPC is used as a combined injectable contraceptive to prevent pregnancy in women under the brand name Injectable No. 1 (or Chinese Injectable No. 1) in China as well.[4][5][6][7] When used as a combined injectable contraceptive, EV/OHPC is given twice in the first month and then once per month thereafter.[7][13][14]
Available forms
EV/OHPC is available for general use (e.g., as Gravibinon) in the form of ampoules containing 5 to 10 mg estradiol valerate (EV) and 250 to 500 mg hydroxyprogesterone caproate (OHPC).[8] It is available for use as a combined injectable contraceptive specifically at a dose of 5 mg EV and 250 mg OHPC.[4][5][6][7]
Side effects
EV/OHPC as a combined injectable contraceptive has a relatively short duration and is associated with a high incidence of menstrual irregularity, for instance polymenorrhea (short and hence fast cycles).[7][13] This may be unacceptable to many women.[7] Twice-monthly administration of half doses has not been found to improve breakthrough bleeding, though cycle length increased to 20 to 24 days.[13]
History
EV/OHPC was reportedly first introduced for medical use in 1955.[15] The medication was developed by Schering and marketed under the brand name Gravibinon for the treatment of habitual abortion in Europe by the late 1960s.[16][17][18]
EV/OHPC was the first combined injectable contraceptive to be studied.[14][7] It was first evaluated by Siegel and colleagues in 1963.[14][7] The doses used in their study were 10 mg EV and 500 mg OHPC.[14] Around the same time as the Siegel study, a half-dose formulation containing 5 mg EV and 250 mg OHPC was developed and subsequently marketed for use in China under the brand name Injectable No. 1 (or Chinese Injectable No. 1).[7][14] The formulation was also reported to be marketed in a few countries neighboring China.[19] EV/OHPC was also studied at the same dose by a "major European pharmaceutical company" in 1971, but was found to produce short menstrual cycles of 17 to 18 days with once-monthly administration and 20 to 24 days with twice-monthly administration.[5] As a result of these menstrual disturbances, the company abandoned development of the formulation.[5]
EV/OHPC was one of only two combined injectable contraceptives to have been marketed by 1976, and was one of only three combined injectable contraceptives with considerable clinical experience by 1976.[20][21] The others were estradiol enanthate/algestone acetophenide (E2-EN/DHPA; brand names Perlutal, Topasel), which had been marketed in Spain and Latin America, and estradiol cypionate/medroxyprogesterone acetate (EC/MPA; code name Cyclo-Provera), which was still experimental by 1976 and did not become formally available for clinical use until the 1990s.[21][7] By 1994, at which point EC/MPA (brand names Cyclofem and later Lunelle) and estradiol valerate/norethisterone enanthate (EV/NETE; brand name Mesigyna) had been introduced, EV/OHPC had been in use for many years.[21][7]
EV/OHPC and E2-EN/DHPA have been referred to as first-generation combined injectable contraceptives, while EC/MPA and EV/NETE have been referred to as second-generation combined injectable contraceptives.[20]
Society and culture
Brand names
EV/OHPC has been marketed under the brand names Deluteval (or Deluteval 2X), Gravibinon, Gravibinan, Gravidinona, and Gestadinona for the treatment of threatened abortion and other general uses.[22][23][24][8][25][26] It has been marketed under the brand name Injectable No. 1 or Chinese Injectable No. 1 for use as a combined injectable contraceptive.[4][5][6]
Availability
EV/OHPC is marketed under the brand names Gravidinona in Mexico and Gestadinona in Brazil for threatened abortion.[22][23][24] It was also marketed under the brand name Gravidinona in Chile but was discontinued in this country.[23][24] EV/OHPC was also marketed under the brand names Gravibinon (Schering) in Austria, Belgium, and Germany and Gravibinan (Schering) in France, Italy, and Turkey for threatened abortion as well, but has been discontinued in these countries.[23][24][8][25][26] EV/OHPC is marketed for use as a combined injectable contraceptive under the brand name Injectable No. 1 or Chinese Injectable No.1 in the China.[4][5][6]
Usage
It was estimated in 1995 that EV/OHPC had been used as a combined injectable contraceptive in China by about 1 million women.[27] However, combined injectable contraceptives like EV/OHPC are unlikely to constitute a large proportion of contraceptive use in the countries in which they are available.[27]
See also
References
- Gunther Göretzlehner; Christian Lauritzen; Ulf Göretzlehner (1 January 2007). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 117, 337, 385, 391–392. ISBN 978-3-11-020864-1.
- Schindler, A. E. (2001). "Behandlung der Risikoschwangerschaft mit Gravibinon®". Zentralblatt für Gynäkologie. 123 (6): 353–356. doi:10.1055/s-2001-16285. ISSN 0044-4197. PMID 11488163.
- Schindler AE (January 2004). "First trimester endocrinology: consequences for diagnosis and treatment of pregnancy failure". Gynecol. Endocrinol. 18 (1): 51–7. doi:10.1080/09513590310001651795. PMID 15106366.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 561–. ISBN 978-3-7692-2114-5.
- Lim CE, Ho KK, Cheng NC, Wong FW (September 2013). "Combined oestrogen and progesterone for preventing miscarriage". Cochrane Database Syst Rev (9): CD009278. doi:10.1002/14651858.CD009278.pub2. hdl:10453/117991. PMID 24068368.
- Gunther Göretzlehner; Christian Lauritzen; Thomas Römer; Winfried Rossmanith (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 227–228. ISBN 978-3-11-024568-4.
- Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- Ulrich, U.; Pfeifer, T.; Buck, G.; Keckstein, J.; Lauritzen, C. (1995). "High-dose estrogen-progestogen injections in gonadal dysgenesis, ovarian hypoplasia, and androgen insensitivity syndrome: Impact on bone density". Adolescent and Pediatric Gynecology. 8 (1): 20–23. doi:10.1016/S0932-8610(12)80156-3. ISSN 0932-8610.
- Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94,99 https://www.popline.org/node/423289 Archived 2019-03-24 at the Wayback Machine
- Paolo Giovanni Artini; Andrea R. Genazzani; Felice Petraglia (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 101–102. ISBN 978-1-84214-071-0.
- Kaiser, R. (2008). "Gestagen-Östrogen-Kombinationen in der Gynäkologie. Zur Geschichte, Dosierung und Anwendung eines Hormonprinzips" [Progestogen-Estrogen Combinations in Gynecology. History, Dosage, and Use of a Hormone Principle]. Geburtshilfe und Frauenheilkunde. 53 (7): 503–513. doi:10.1055/s-2007-1022924. ISSN 0016-5751.
Zur kombinierten Anwendung von Gestagen en und Östrogenen stand en zunächst ölgelöstes Östradiolbenzoat und Progesteron zur Verfügung. Das erste derartige Mischpräparat kam in Deutschland 1950 auf den Mark t. Dem Wunsch nach verlän gerter Wirkungsdauer entsprach en dann Kristallmischsuspension en verschiedener Korngröße aus Östradiolmonobenzoat + Progesteron, deren Anwendung sich auf klinische Untersuchungen besch ränkte (83). Ölgelöste Depotpräparate mit Östradiolbenzoat oder -valerat + 17-hydroxyprogesteroncaproat wurden ab 1955 in die Therapie eingeführt (45.46).
- Acta Europaea Fertilitatis. Morgagni Edizioni Scientifiche. 1969. p. 662,665.
- Belgium (1969). Belgisch staatsblad. p. 4798.
- Pundel JP (1971). "[Prolonged amenorrhea after abortion during intensive treatment with progestagens]". Gynecol Prat (in French). 22 (2): 77–85. PMID 5562318.
- "Facts about once-a-month injectable contraceptives: memorandum from a WHO meeting". Bull. World Health Organ. 71 (6): 677–89. 1993. PMC 2393537. PMID 8313486.
- J. Bringer; B. Hedon (15 September 1995). Fertility and Sterility: A Current Overview. CRC Press. pp. 47–. ISBN 978-1-85070-694-6.
- Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- https://www.drugs.com/international/hydroxyprogesterone.html
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- http://www.micromedexsolutions.com/micromedex2/librarian/
- PDR Generics. Medical Economics. 1996. p. 1171. ISBN 978-1-56363-151-1.
- Axel Kleemann; Jürgen Engel (2001). Pharmaceutical Substances: Syntheses, Patents, Applications. Thieme. p. 1033. ISBN 978-3-13-558404-1.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.