Levonorgestrel acetate
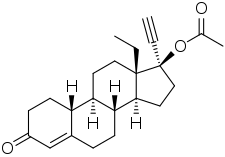
Levonorgestrel acetate (LNG-A), or levonorgestrel 17β-acetate, also known as 3-ketonorgestimate, is a progestin which was never marketed.[1][2][3][4] It is a progestogen ester and is the C17β acetate ester and a prodrug of levonorgestrel.[2] Norgestimate is the C3 oxime of LNG-A.[1][5] The drug is a minor active metabolite of norgestimate, which is a prodrug of norelgestromin and to a lesser extent of levonorgestrel and LNG-A.[2] LNG-A has high affinity for the progesterone receptor, about 135% of that of promegestone (relative to 150% for levonorgestrel).[2][3] Along with levonorgestrel butanoate, LNG-A was investigated as a hormonal contraceptive by the Population Council.[6][4]
 | |
| Clinical data | |
|---|---|
| Other names | LNG-A; LNGA; 3-Ketonorgestimate; Levonorgestrel 17β-acetate; D-(–)-Norgestrel 17β-acetate; 17α-Ethynyl-18-methylestr-4-en-17β-ol-3-one 17β-acetate; 17β-Acetoxy-13β-ethyl-17α-ethynylgon-4-en-3-one |
| Drug class | Progestogen; Progestogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.123.252 |
| Chemical and physical data | |
| Formula | C23H30O3 |
| Molar mass | 354.49 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Naderi S, Fotherby K (1983). "Long-acting contraceptive agents: in vitro hydrolysis of esters of norethisterone and levonorgestrel". Steroids. 41 (3): 397–417. doi:10.1016/0039-128x(83)90110-1. PMID 6419410.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins". Maturitas. 61 (1–2): 171–80. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889.
- Alvarez-Sanchez F, Brache V, Jackanicz T, Faundes A (1992). "Evaluation of four different contraceptive vaginal rings: steroid serum levels, luteal activity, bleeding control and lipid profiles". Contraception. 46 (4): 387–98. doi:10.1016/0010-7824(92)90101-x. PMID 1486777.
- Sven O. Skouby (15 July 1997). Clinical Perspectives on a New Gestodene Oral Contraceptive Containing 20μg of Ethinylestradiol. CRC Press. pp. 11–. ISBN 978-1-85070-786-8.
- Population Reports: Injectables and implants. Department of Medical and Public Affairs, George Washington University. 1987.
The Population Council also plans to test vaginal rings with two other progestins, ST-1435 and levonorgestrel acetate, alone and combined with ethinyl estradiol (168).
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.