SN-22
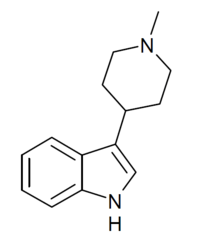
SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19nM at 5HT2 subtypes vs 514 nM at 5-HT1A receptors.[1] Many related derivatives are known, most of which are ligands for 5-HT1A, 5-HT6 or dopamine D2 receptors or show SSRI activity.[2][3][4][5][6]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H18N2 |
| Molar mass | 214.306 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- BRL-54443
- LY-334370
- MPTP
- Naratriptan
- RU-24969
- RU-27592
References
- Taylor, E. W.; Nikam, S. S.; Lambert, G.; Martin, A. R.; Nelson, D. L. (1988). "Molecular determinants for recognition of RU 24969 analogs at central 5-hydroxytryptamine recognition sites: Use of a bilinear function and substituent volumes to describe steric fit". Molecular Pharmacology. 34 (1): 42–53. PMID 3393140.
- Agarwal, Atul; Pearson, Philip P.; Taylor, Ethan Will; Li, Hong B.; Dahlgren, Torsten; Herslof, Margareta; Yang, Youhua; Lambert, Georgina; Nelson, David L. (1993). "Three-dimensional quantitative structure-activity relationships of 5-HT receptor binding data for tetrahydropyridinylindole derivatives: A comparison of the Hansch and CoMFA methods". Journal of Medicinal Chemistry. 36 (25): 4006–4014. doi:10.1021/jm00077a003.
- Cole, Derek C.; Ellingboe, John W.; Lennox, William J.; Mazandarani, Hossein; Smith, Deborah L.; Stock, Joseph R.; Zhang, Guoming; Zhou, Ping; Schechter, Lee E. (2005). "N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 15 (2): 379–383. doi:10.1016/j.bmcl.2004.10.064. PMID 15603958.
- Deskus, Jeffrey A.; Epperson, James R.; Sloan, Charles P.; Cipollina, Joseph A.; Dextraze, Pierre; Qian-Cutrone, Jingfang; Gao, Qi; Ma, Baoqing; Beno, Brett R.; Mattson, Gail K.; Molski, Thaddeus F.; Krause, Rudolph G.; Taber, Matthew T.; Lodge, Nicholas J.; Mattson, Ronald J. (2007). "Conformationally restricted homotryptamines 3. Indole tetrahydropyridines and cyclohexenylamines as selective serotonin reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters. 17 (11): 3099–3104. doi:10.1016/j.bmcl.2007.03.040. PMID 17391962.
- Mattsson, Cecilia; Andreasson, Theresa; Waters, Nicholas; Sonesson, Clas (2012). "Systematic in Vivo Screening of a Series of 1-Propyl-4-arylpiperidines against Dopaminergic and Serotonergic Properties in Rat Brain: A Scaffold-Jumping Approach". Journal of Medicinal Chemistry. 55 (22): 9735–9750. doi:10.1021/jm300975f. PMID 23043306.
- US 6046215, "Inhibition of serotonin reuptake"
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.