Kavalactone
Kavalactones are a class of lactone compounds found in the kava shrub. Kavalactones are under research for potential to have various psychotropic effects, including anxiolytic and sedative/hypnotic activities.
Enzyme inhibition
Kava extract has been shown to potently inhibit a wide range of hepatic enzymes, suggesting a very high potential for interactions with many pharmaceuticals and herbal medications.[1]
Research
Several preliminary studies are assessing potential effects of kava, including its anxiolytic actions[2] and hepatotoxicity, but the role specifically of kavalactones among many other kava compounds for these effects remains under study.[3][4]
Toxicity
Several kavalactones (e.g.methysticin and yangonin) have been reported to affect a group of enzymes involved in metabolism, called CYP1A1. Hepatotoxicity has been reported in a small portion of previously healthy kava users,[3][5] particularly of extracts as opposed to whole root powders.[6]
Numerous kavalactones have apoptotic effects on various human tissues, a mechanism under preliminary study for the toxic effects of kava use.[7]
Compounds
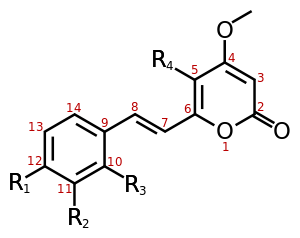
At least 18 different kavalactones have been identified to date, with methysticin being the first identified.[8] Multiple analogues, such as ethysticin, have also been isolated.[9] Some consist of a substituted α-pyrone as the lactone while others are partially saturated.
The average elimination half-life of kavalactones typically present in kava root is 9 hr.[10]
| Name | Structure | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| Yangonin | 1 | -OCH3 | -H | -H | -H |
| 10-methoxyyangonin | 1 | -OCH3 | -H | -OCH3 | -H |
| 11-methoxyyangonin | 1 | -OCH3 | -OCH3 | -H | -H |
| 11-hydroxyyangonin | 1 | -OCH3 | -OH | -H | -H |
| Desmethoxyyangonin | 1 | -H | -H | -H | -H |
| 11-methoxy-12-hydroxydehydrokavain | 1 | -OH | -OCH3 | -H | -H |
| 7,8-dihydroyangonin | 2 | -OCH3 | -H | -H | -H |
| Kavain | 3 | -H | -H | -H | -H |
| 5-hydroxykavain | 3 | -H | -H | -H | -OH |
| 5,6-dihydroyangonin | 3 | -OCH3 | -H | -H | -H |
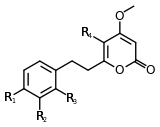
| 7,8-dihydrokavain | 4 | -H | -H | -H | -H |
| 5,6,7,8-tetrahydroyangonin | 4 | -OCH3 | -H | -H | -H |
| 5,6-dehydromethysticin | 5 | -O-CH2-O- | -H | -H | |
| Methysticin | 7 | -O-CH2-O- | -H | -H | |
| 7,8-dihydromethysticin | 8 | -O-CH2-O- | -H | -H | |
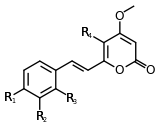 Structure 1 |
 Structure 2 |
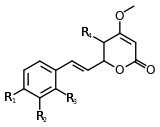 Structure 3 |
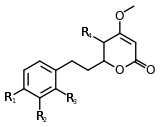 Structure 4 |
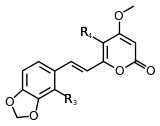 Structure 5 |
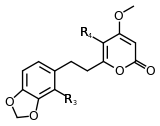 Structure 6 |
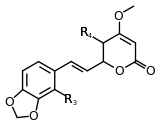 Structure 7 |
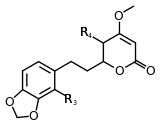 Structure 8 |
Biosynthesis
The kavalactone biosynthetic pathway in Piper methysticum was described in 2019.[11]
See also
References
- James M. Mathews; Amy S. Etheridge; Sherry R. Black (2002). "Inhibition of Human Cytochrome P450 Activities by Kava Extract and Kavalactones". Drug Metabolism and Disposition. 30 (11): 1153–1157. doi:10.1124/dmd.30.11.1153.
- Sarris, Jerome; LaPorte, Emma; Schweitzer, Isaac (2011-01-01). "Kava: A Comprehensive Review of Efficacy, Safety, and Psychopharmacology". Australian & New Zealand Journal of Psychiatry. 45 (1): 27–35. doi:10.3109/00048674.2010.522554.
- Teschke, R; Lebot, V (2011). "Proposal for a kava quality standardization code". Food and Chemical Toxicology. 49 (10): 2503–16. doi:10.1016/j.fct.2011.06.075. PMID 21756963.
- Wang, J; Qu, W; Bittenbender, H. C.; Li, Q. X. (2013). "Kavalactone content and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of kavalactones using different solvents". Journal of Food Science and Technology. 52 (2): 1164–1169. doi:10.1007/s13197-013-1047-2. PMC 4325077. PMID 25694734.
- Teschke, R; Qiu, S. X.; Xuan, T. D.; Lebot, V (2011). "Kava and kava hepatotoxicity: Requirements for novel experimental, ethnobotanical and clinical studies based on a review of the evidence". Phytotherapy Research. 25 (9): 1263–74. doi:10.1002/ptr.3464. PMID 21442674.
- AC Brown (2007). "Traditional kava beverage consumption and liver function tests in a predominantly Tongan population in Hawaii revealed no liver impairment". Archived from the original on 2012-03-07. Retrieved 2009-03-17.
- Tang, J; Dunlop, RA; Rowe, A; Rodgers, KJ; Ramzan, I (2010). "Kavalactones Yangonin and Methysticin Induce Apoptosis in Human Hepatocytes (HepG2) In Vitro". Phytotherapy Research. 25 (3): 417–23. doi:10.1002/ptr.3283. PMID 20734326.
- Naumov, P.; Dragull, K.; Yoshioka, M.; Tang, C.-S.; Ng, S. W. (2008). "Structural Characterization of Genuine (-)-Pipermethystine, (-)-Epoxypipermethystine, (+)-Dihydromethysticin and Yangonin from the Kava Plant (Piper methysticum)". Natural Product Communications. 3 (8): 1333–1336.
- Shulgin, A. (1973). "The narcotic pepper - the chemistry and pharmacology of Piper methysticum and related species". Bulletin on Narcotics (2): 59–74.
- "Kava (Piper methysticum): Pharmacodynamics/Kinetics". Sigma-Aldrich Co. LLC. 2010.
- Pluskal, Tomáš; Torrens-Spence, Michael P.; Fallon, Timothy R.; De Abreu, Andrea; Shi, Cindy H.; Weng, Jing-Ke (2019-07-22). "The biosynthetic origin of psychoactive kavalactones in kava". Nature Plants. Springer Science and Business Media LLC. 5 (8): 867–878. doi:10.1038/s41477-019-0474-0. ISSN 2055-0278.