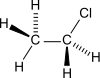
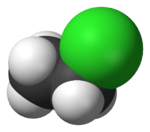
Chloroethane
Chloroethane, commonly known by its old name ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.[9]
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chloroethane | |||
| Other names
Ethyl chloride, Monochloroethane, Chlorene, Muriatic ether, EtCl, UN 1037, Hydrochloric ether | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.755 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C2H5Cl | ||
| Molar mass | 64.51 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Pungent, ethereal[1] | ||
| Density | 0.921 g/cm3 (0-4 °C)[2] 0.8898 g/cm3 (25 °C) | ||
| Melting point | −138.7 °C (−217.7 °F; 134.5 K) | ||
| Boiling point | 12.27 °C (54.09 °F; 285.42 K) decomposes at 510 °C[3] | ||
Solubility in water |
0.447 g/100 mL (0 °C) 0.574 g/100 mL (20 °C)[4][3] | ||
| Solubility | Soluble in alcohol, ether[5] | ||
| Solubility in ethanol | 48.3 g/100 g (21 °C)[3] | ||
| Vapor pressure | 8.4 kPa (-40 °C) 62.3 kPa (0 °C)[6] 134.6 kPa (20 °C)[1] | ||
Henry's law constant (kH) |
11.1 L·atm/mol (24 °C)[1] | ||
Refractive index (nD) |
1.3676 (20 °C) 1.001 (25 °C)[1] | ||
| Viscosity | 0.279 cP (10 °C)[1] | ||
| Structure | |||
Dipole moment |
2.06 D | ||
| Thermochemistry | |||
Heat capacity (C) |
104.3 J/mol·K[3] | ||
Std molar entropy (S |
275.7 J/mol·K[3] | ||
Std enthalpy of formation (ΔfH⦵298) |
-137 kJ/mol[3][6] | ||
Gibbs free energy (ΔfG˚) |
-59.3 kJ/mol[3] | ||
| Pharmacology | |||
| N01BX01 (WHO) | |||
| Hazards | |||
| Main hazards | Flammable | ||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H220, H280, H351, H412[2] | ||
GHS precautionary statements |
P210, P273, P281, P410+403[2] | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −43 °C (−45 °F; 230 K) open cup[4] −50 °C (−58 °F; 223 K) closed cup[2][5] | ||
Autoignition temperature |
494 to 519 °C (921 to 966 °F; 767 to 792 K)[3][5] | ||
| Explosive limits | 3.8%-15.4%[7] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
59,701 ppm (rat, 2 hr) 54,478 ppm (mouse, 2 hr) [8] | ||
LCLo (lowest published) |
40,000 ppm (guinea pig, 45 min)[8] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1000 ppm (2600 mg/m3)[7] | ||
REL (Recommended) |
Handle with caution in the workplace.[7] | ||
IDLH (Immediate danger) |
3800 ppm[7] | ||
| Related compounds | |||
Related haloalkanes |
1,1-dichloroethane 1,2-dichloroethane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
Chloroethane is produced by hydrochlorination of ethylene:[9]
- C2H4 + HCl → C2H5Cl
At various times in the past, chloroethane has also been produced from ethanol and hydrochloric acid, or from ethane and chlorine, but these routes are no longer economical. Some chloroethane is generated as a byproduct of polyvinyl chloride production. Should demand for chloroethane continue to fall to the point where making it for its own sake is not economical, this may become the leading source of the chemical.
Uses
Ethyl chloride is an inexpensive ethylating agent. It reacts with aluminium metal to give ethylaluminium sesquichloride, a precursor to polymers and other useful organoaluminium compounds.[10] Chloroethane is used to convert cellulose to ethylcellulose, a thickening agent and binder in paints, cosmetics, and similar products.
Like other chlorinated hydrocarbons, chloroethane has been used as a refrigerant, an aerosol spray propellant, an anesthetic, and a blowing agent for foam packaging. For a time it was used as a promoter chemical in the aluminium chloride catalyzed process to produce ethylbenzene, the precursor for styrene monomer. At present though, it is not widely used in any of these roles.
Obsolete uses
Beginning in 1922 and continuing through most of the 20th century, the major use of chloroethane was to produce tetraethyllead (TEL), an anti-knock additive for gasoline. TEL has been or is being phased out in most of the industrialized world, and the demand for chloroethane has fallen sharply.[9]
Niche uses
It acts as a mild topical anesthetic by its chilling effect when sprayed on skin, such as when removing splinters or incising abscesses in a clinical setting. It was standard equipment in “casualty” wards. It was commonly used to induce general anaesthesia before continuing with di-ethyl ether, which had a very much slower up-take.
The heat absorbed by the boiling liquid on tissues produces a deep and rapid chill, but since the boiling point is well above the freezing point of water, it presents no danger of frostbite.
In dentistry, chloroethane is used as one of the means of diagnosing a 'dead tooth', i.e. one in which the pulp has died. A small amount of the substance is placed on the suspect tooth using a cotton wad. Chloroethane's low boiling point creates a localised chilling effect. If the tooth is still alive this should be sensed by the patient as mild discomfort that subsides when the wad is removed.
Safety
The vapor is flammable and narcotic, which requires care.
Monochloroethane is the least toxic of the chloroethanes. Like other chlorinated hydrocarbons, it is a central nervous system depressant, albeit a less potent one than many similar compounds. People breathing its vapors at less than 1% concentration in air usually experience no symptoms. At concentrations of 3% to 5%, victims usually exhibit symptoms similar to those of alcohol intoxication. Breathing its vapors at >15% concentration is often fatal but most commercially available handheld containers contain a total of 30% per volume of concentrated vapors which naturally disperse in the outside air.
If exposed to concentrations higher than 6% to 8% victims often exhibit shallow breathing, loss of consciousnesses, and depressed heart-rate. They can be aroused (brought around) with physical contact or loud noise. At this point removal from the area of exposure is advised to restore consciousness. The long-term effects of exposure over a period of 4 or more hours will cause side effects similar to alcoholic hang-over with dehydration, dizziness, loss of clear vision and temporary loss of consciousness, which can last an hour or more. If no longer exposed to the gas, a victim will return to normal health quickly. This can be helped with intake of extra fluids, vitamins, and sugars.
Toxic over-exposure starts at 9% to 12% concentrations, the heart rate drops further, the victim may have more shallow breathing or stop all together, they do not respond to any outside stimulation and may begin to involuntarily gasp, belch or vomit, which can lead to aspiration if the victim is not turned on his side. This constitutes a medical emergency and requires prompt action. It is advised to move the victim to clear air and administer forced breathing for them to purge the lungs of the toxic fumes. If the victim recovers quickly enough, hospitalization may not be required, but will require a medical examination to ensure that no organ damage has occurred.
At >12% concentration, the victim's heart, lungs and kidneys begin to fail. Immediate CPR followed by medical support measures may be required to prevent fatal kidney, lung and heart failure.
Studies on the effects of chronic ethyl chloride exposure in animals have given inconsistent results, and no data exists for its long-term effects on humans. Some studies have reported that prolonged exposure can produce liver or kidney damage, or uterine cancer in mice, but these data have been difficult to reproduce.
While chloroethane is not classified as to carcinogenicity to humans specifically,[11] recent information suggests carcinogenic potential and it has been designated as ACGIH category A3, Confirmed Animal Carcinogen with Unknown Relevance to Humans. As a result, the U.S. State of California has incorporated it into Proposition 65 as a known carcinogen. Nonetheless, it is still used in medicine as a local anesthetic.[12]
Recreational drug
Chloroethane is a recreational inhalant drug, although it should not be confused with a duster or canned air, which is composed of fluorinated low-weight hydrocarbons such as tetrafluoromethane, chlorodifluoromethane or another similar gas. Similar to poppers, ethyl chloride is used as an inhalant (huffed) during sexual activity, primarily by homosexual men. In Brazil, it is a traditional (though illegal) drug taken during Carnaval parades, known as "lança-perfume", which is a high concentration of chloroethane. [13]
References
- CID 6337 from PubChem
- Sigma-Aldrich Co., Chloroethane. Retrieved on 2014-05-26.
- http://chemister.ru/Database/properties-en.php?dbid=1&id=1545
- "Summary of Emissions Associated with Sources of Ethyl Chloride". nepis.epa.gov. National Service Center for Environmental Publications (NSCEP). Retrieved 2014-05-26.
- "Material Safety Data Sheet" (PDF). www.mathesongas.com. Matheson Tri-Gas, Inc. Retrieved 2014-05-26.
- Ethyl chloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-26)
- NIOSH Pocket Guide to Chemical Hazards. "#0267". National Institute for Occupational Safety and Health (NIOSH).
- "Ethyl chloride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E. L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack (2006). "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2.
- Krause, M.J.; Orlandi, F.; Saurage, A.T.; Zietz, Jr., J.R. (2000). "Aluminum Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_543.
- Chloroethane, IARC
- http://healthland.time.com/2010/12/02/whats-lanca-perfume-the-biggest-drug-in-rio-youve-never-heard-of/
External links
| Wikimedia Commons has media related to Chloroethane. |
- International Chemical Safety Card 0132
- NIOSH Pocket Guide to Chemical Hazards. "#0267". National Institute for Occupational Safety and Health (NIOSH).
- IARC Monograph "Chloroethane."
- Ethyl chloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov
- National Pollutant Inventory - Chloroethane