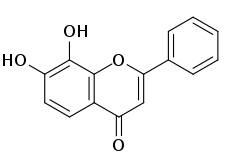
7,8-Dihydroxyflavone
7,8-Dihydroxyflavone (7,8-DHF) is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves.[2][3][4] It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB) (Kd ≈ 320 nM), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF).[5][6][7] 7,8-DHF is both orally bioavailable and able to penetrate the blood–brain barrier.[8][9] A prodrug of 7,8-DHF with greatly improved potency and pharmacokinetics, R13 (and, formerly, R7), is under development for the treatment of Alzheimer's disease.[10][11]
 | |
| Clinical data | |
|---|---|
| Other names | 7,8-DHF |
| Pharmacokinetic data | |
| Bioavailability | ~5% (in mice)[1] |
| Elimination half-life | < 30 minutes (in mice)[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.903 |
| Chemical and physical data | |
| Formula | C15H10O4 |
| Molar mass | 254.238 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
7,8-DHF has demonstrated therapeutic efficacy in animal models of a variety of central nervous system disorders,[7] including depression,[8] Alzheimer's disease,[12][13][14] cognitive deficits in schizophrenia,[15] Parkinson's disease,[5] Huntington's disease,[16] amyotrophic lateral sclerosis,[17] traumatic brain injury,[18] cerebral ischemia,[19][20] fragile X syndrome,[21] and Rett syndrome.[22] 7,8-DHF also shows efficacy in animal models of age-associated cognitive impairment[23] and enhances memory consolidation and emotional learning in healthy rodents.[24][25] In addition, 7,8-DHF possesses powerful antioxidant activity independent of its actions on the TrkB receptor,[26] and protects against glutamate-induced excitotoxicity,[27] 6-hydroxydopamine-induced dopaminergic neurotoxicity,[28] and oxidative stress-induced genotoxicity.[29] It was also found to block methamphetamine-induced dopaminergic neurotoxicity, an effect which, in contrast to the preceding, was found to be TrkB-dependent.[30]
In 2017, evidence was published suggesting that 7,8-DHF and various other reported small-molecule TrkB agonists might not actually be direct agonists of the TrkB and might be mediating their observed effects by other means.[31][32]
7,8-DHF has been found to act as a weak aromatase inhibitor in vitro (Ki = 10 μM),[33] though there is evidence to suggest that this might not be the case in vivo.[5] In addition, it has been found to inhibit aldehyde dehydrogenase and estrogen sulfotransferase in vitro (Ki = 35 μM and 1–3 μM, respectively), though similarly to the case of aromatase, these activities have not yet been confirmed in vivo.[5] Unlike many other flavonoids, 7,8-DHF does not show any inhibitory activity on 17β-hydroxysteroid dehydrogenase.[34] 7,8-DHF has also been observed to possess in vitro antiestrogenic effects at very high concentrations (Ki = 50 μM).[35][36]
A variety of close structural analogues of 7,8-DHF have also been found to act as TrkB agonists in vitro, including diosmetin (5,7,3'-trihydroxy-4'-methoxyflavone), norwogonin (5,7,8-trihydroxyflavone), 4'-dimethylamino-7,8-dihydroxyflavone (4'-DMA-7,8-DHF), 7,8,3'-trihydroxyflavone, 7,3'-dihydroxyflavone, 7,8,2'-trihydroxyflavone, 3,7,8,2'-tetrahydroxyflavone, and 3,7-dihydroxyflavone.[37] The highly hydroxylated analogue gossypetin (3,5,7,8,3',4'-hexahydroxyflavone), conversely, appears to be an antagonist of TrkB in vitro.[37]
See also
- List of investigational antidepressants
- Tropomyosin receptor kinase B § Agonists
7,8-Dihydroxyflavone was also found to decrease mouse sleep in dark phase and reduce hypothalamus level of orexin A (Feng et al 2015).
References
- US application 20150274692, Keqiang Ye, "7,8-Dihydoxyflavone and 7,8-substituted flavone derivatives, compositions, and methods related thereto", published 2015-10-01, assigned to Emory University
- Andero, R.; Ressler, K.J. (2012). "Fear extinction and BDNF: translating animal models of PTSD to the clinic". Genes, Brain and Behavior. 11 (5): 503–512. doi:10.1111/j.1601-183X.2012.00801.x. ISSN 1601-1848. PMC 3389160. PMID 22530815.
- Colombo, Paola S.; Flamini, Guido; Christodoulou, Michael S.; Rodondi, Graziella; Vitalini, Sara; Passarella, Daniele; Fico, Gelsomina (2014). "Farinose alpine Primula species: Phytochemical and morphological investigations" (PDF). Phytochemistry. 98: 151–159. doi:10.1016/j.phytochem.2013.11.018. ISSN 0031-9422. PMID 24345641.
- Cell Press (2015). "Molecule found in tree leaves helps female mice combat weight gain; males unaffected". ScienceDaily. Retrieved 2015-03-19.
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K (2010). "A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone". Proc. Natl. Acad. Sci. U.S.A. 107 (6): 2687–92. doi:10.1073/pnas.0913572107. PMC 2823863. PMID 20133810.
- Liu X, Obianyo O, Chan CB, Huang J, Xue S, Yang JJ, Zeng F, Goodman M, Ye K (2014). "Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor". J. Biol. Chem. 289 (40): 27571–84. doi:10.1074/jbc.M114.562561. PMC 4183797. PMID 25143381.
- Zeng Y, Wang X, Wang Q, Liu S, Hu X, McClintock SM (2013). "Small molecules activating TrkB receptor for treating a variety of CNS disorders". CNS Neurol Disord Drug Targets. 12 (7): 1066–77. doi:10.2174/18715273113129990089. PMID 23844685.
- Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, Phun LH, France S, Xiao G, Jia Y, Luo HR, Ye K (2010). "A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect". J. Med. Chem. 53 (23): 8274–86. doi:10.1021/jm101206p. PMC 3150605. PMID 21073191.
- Liu X, Chan CB, Qi Q, Xiao G, Luo HR, He X, Ye K (2012). "Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression". J. Med. Chem. 55 (19): 8524–37. doi:10.1021/jm301099x. PMC 3491656. PMID 22984948.
- Chen C, Wang Z, Zhang Z, Liu X, Kang SS, Zhang Y, Ye K (January 2018). "The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer's disease". Proc. Natl. Acad. Sci. U.S.A. 115 (3): 578–583. doi:10.1073/pnas.1718683115. PMC 5777001. PMID 29295929.
- Liu, Chaoyang; Chan, Chi Bun; Ye, Keqiang (2016). "7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders". Translational Neurodegeneration. 5 (1): 2. doi:10.1186/s40035-015-0048-7. ISSN 2047-9158. PMC 4702337. PMID 26740873.
- Castello NA, Nguyen MH, Tran JD, Cheng D, Green KN, LaFerla FM (2014). "7,8-Dihydroxyflavone, a small molecule TrkB agonist, improves spatial memory and increases thin spine density in a mouse model of Alzheimer disease-like neuronal loss". PLoS ONE. 9 (3): e91453. doi:10.1371/journal.pone.0091453. PMC 3948846. PMID 24614170.
- Chen C, Li XH, Zhang S, Tu Y, Wang YM, Sun HT (2014). "7,8-dihydroxyflavone ameliorates scopolamine-induced Alzheimer-like pathologic dysfunction". Rejuvenation Res. 17 (3): 249–54. doi:10.1089/rej.2013.1519. PMID 24325271.
- Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, Weinshenker D, Ye K (2014). "7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer's disease". Neuropsychopharmacology. 39 (3): 638–50. doi:10.1038/npp.2013.243. PMC 3895241. PMID 24022672.
- Yang YJ, Li YK, Wang W, Wan JG, Yu B, Wang MZ, Hu B (2014). "Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia". Pharmacol. Biochem. Behav. 122: 30–6. doi:10.1016/j.pbb.2014.03.013. PMID 24662915.
- Jiang M, Peng Q, Liu X, Jin J, Hou Z, Zhang J, Mori S, Ross CA, Ye K, Duan W (2013). "Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington's disease". Hum. Mol. Genet. 22 (12): 2462–70. doi:10.1093/hmg/ddt098. PMC 3658168. PMID 23446639.
- Korkmaz OT, Aytan N, Carreras I, Choi JK, Kowall NW, Jenkins BG, Dedeoglu A (2014). "7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis". Neurosci. Lett. 566: 286–91. doi:10.1016/j.neulet.2014.02.058. PMC 5906793. PMID 24637017.
- Wu CH, Hung TH, Chen CC, Ke CH, Lee CY, Wang PY, Chen SF (2014). "Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling". PLoS ONE. 9 (11): e113397. doi:10.1371/journal.pone.0113397. PMC 4240709. PMID 25415296.
- Wang B, Wu N, Liang F, Zhang S, Ni W, Cao Y, Xia D, Xi H (2014). "7,8-dihydroxyflavone, a small-molecule tropomyosin-related kinase B (TrkB) agonist, attenuates cerebral ischemia and reperfusion injury in rats". J. Mol. Histol. 45 (2): 129–40. doi:10.1007/s10735-013-9539-y. PMID 24045895.
- Uluc K, Kendigelen P, Fidan E, Zhang L, Chanana V, Kintner D, Akture E, Song C, Ye K, Sun D, Ferrazzano P, Cengiz P (2013). "TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia". CNS Neurol Disord Drug Targets. 12 (3): 360–70. doi:10.2174/18715273113129990061. PMC 3674109. PMID 23469848.
- Tian M, Zeng Y, Hu Y, Yuan X, Liu S, Li J, Lu P, Sun Y, Gao L, Fu D, Li Y, Wang S, McClintock SM (2015). "7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome". Neuropharmacology. 89: 43–53. doi:10.1016/j.neuropharm.2014.09.006. PMID 25229717.
- Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, Mitchell GS, Chang Q (2012). "7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome". J. Appl. Physiol. 112 (5): 704–10. doi:10.1152/japplphysiol.01361.2011. PMC 3643819. PMID 22194327.
- Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q (2012). "7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats". J. Neurochem. 122 (4): 800–11. doi:10.1111/j.1471-4159.2012.07830.x. PMID 22694088.
- Bollen E, Vanmierlo T, Akkerman S, Wouters C, Steinbusch HM, Prickaerts J (2013). "7,8-Dihydroxyflavone improves memory consolidation processes in rats and mice". Behav. Brain Res. 257: 8–12. doi:10.1016/j.bbr.2013.09.029. PMID 24070857.
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ (2011). "Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning". Am J Psychiatry. 168 (2): 163–72. doi:10.1176/appi.ajp.2010.10030326. PMC 3770732. PMID 21123312.
- Foti, Mario; Piattelli, Mario; Baratta, Maria Tiziana; Ruberto, Giuseppe (1996). "Flavonoids, Coumarins, and Cinnamic Acids as Antioxidants in a Micellar System. Structure−Activity Relationship†". Journal of Agricultural and Food Chemistry. 44 (2): 497–501. doi:10.1021/jf950378u. ISSN 0021-8561.
- Chen J, Chua KW, Chua CC, Yu H, Pei A, Chua BH, Hamdy RC, Xu X, Liu CF (2011). "Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity". Neurosci. Lett. 499 (3): 181–5. doi:10.1016/j.neulet.2011.05.054. PMID 21651962.
- Han X, Zhu S, Wang B, Chen L, Li R, Yao W, Qu Z (2014). "Antioxidant action of 7,8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity". Neurochem. Int. 64: 18–23. doi:10.1016/j.neuint.2013.10.018. PMID 24220540.
- Zhang R, Kang KA, Piao MJ, Ko DO, Wang ZH, Chang WY, You HJ, Lee IK, Kim BJ, Kang SS, Hyun JW (2009). "Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity". Biol. Pharm. Bull. 32 (2): 166–71. doi:10.1248/bpb.32.166. PMID 19182370.
- Ren Q, Zhang JC, Ma M, Fujita Y, Wu J, Hashimoto K (2014). "7,8-Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine". Psychopharmacology. 231 (1): 159–66. doi:10.1007/s00213-013-3221-7. PMID 23934209.
- Boltaev U, Meyer Y, Tolibzoda F, Jacques T, Gassaway M, Xu Q, Wagner F, Zhang YL, Palmer M, Holson E, Sames D (2017). "Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists". Sci Signal. 10 (493): eaal1670. doi:10.1126/scisignal.aal1670. PMID 28831019.
- Todd D, Gowers I, Dowler SJ, Wall MD, McAllister G, Fischer DF, Dijkstra S, Fratantoni SA, van de Bospoort R, Veenman-Koepke J, Flynn G, Arjomand J, Dominguez C, Munoz-Sanjuan I, Wityak J, Bard JA (2014). "A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington's disease". PLoS ONE. 9 (2): e87923. doi:10.1371/journal.pone.0087923. PMC 3913682. PMID 24503862.
- Kao YC, Zhou C, Sherman M, Laughton CA, Chen S (1998). "Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study". Environ. Health Perspect. 106 (2): 85–92. doi:10.1289/ehp.9810685. PMC 1533021. PMID 9435150.
- Le Bail, J.C; Laroche, T; Marre-Fournier, F; Habrioux, G (1998). "Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids". Cancer Letters. 133 (1): 101–106. doi:10.1016/S0304-3835(98)00211-0. ISSN 0304-3835. PMID 9929167.
- Le Bail JC, Varnat F, Nicolas JC, Habrioux G (1998). "Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids". Cancer Lett. 130 (1–2): 209–16. doi:10.1016/S0304-3835(98)00141-4. PMID 9751276.
- Pouget C, Lauthier F, Simon A, Fagnere C, Basly JP, Delage C, Chulia AJ (2001). "Flavonoids: structural requirements for antiproliferative activity on breast cancer cells". Bioorg. Med. Chem. Lett. 11 (24): 3095–7. doi:10.1016/S0960-894X(01)00617-5. PMID 11720850.
- Liu, Xia; Chan, Chi-Bun; Jang, Sung-Wuk; Pradoldej, Sompol; Huang, Junjian; He, Kunyan; Phun, Lien H.; France, Stefan; Xiao, Ge; Jia, Yonghui; Luo, Hongbo R.; Ye, Keqiang (2010). "A Synthetic 7,8-Dihydroxyflavone Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect". Journal of Medicinal Chemistry. 53 (23): 8274–8286. doi:10.1021/jm101206p. ISSN 0022-2623. PMC 3150605. PMID 21073191.